Nitrogen-15 nuclear magnetic resonance spectroscopy
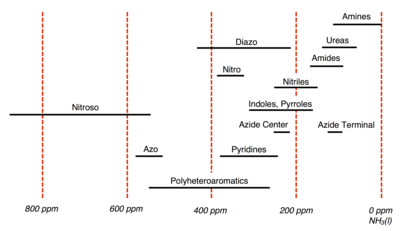
The International Union of Pure and Applied Chemistry (IUPAC) recommends using CH3NO2 as the experimental standard; however in practice many spectroscopists utilize pressurized NH3(l) instead.
A famous application in organic synthesis is to utilize 15N to monitor tautomerization equilibria in heteroaromatics because of the dramatic change in 15N shifts between tautomers.
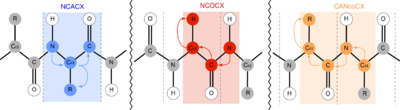
In solid-state nuclear magnetic resonance (ssNMR), for example, 15N is most commonly utilized in NCACX, NCOCX, and CANcoCX pulse sequences.
15N NMR is the most effective method for investigation of structure of heterocycles with a high content of nitrogen atoms (tetrazoles, triazines and their annelated analogs).
The INEPT is an elegant solution in most cases because it increases the Boltzmann polarization and lowers T1 values (thus scans are shorter).
Additionally, INEPT can accommodate negative gyromagnetic ratios, whereas the common nuclear Overhauser effect (NOE) cannot.