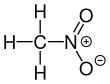
Nitromethane
As an organic solvent, nitromethane has an unusual combination of properties: highly polar (εr = 36 at 20 °C and μ = 3.5 Debye) but aprotic and weakly basic.
[16] Nitric oxide contributes to air pollution, acid rain, and ozone layer depletion.
[citation needed] This analysis indicates that nitromethane generates about 2.3 times the power of gasoline when combined with a given amount of oxygen.
[citation needed] Nitromethane can also be used as a monopropellant, i.e., a propellant that decomposes to release energy without added oxygen.
[20] The following equation describes this process: Nitromethane has a laminar combustion velocity of approximately 0.5 m/s, somewhat higher than gasoline, thus making it suitable for high-speed engines.
These gases often ignite, sometimes spectacularly, as the normally very rich mixtures of the still burning fuel exits the exhaust ports.
Very rich mixtures are necessary to reduce the temperature of combustion chamber hot parts in order to control pre-ignition and subsequent detonation.
[citation needed] A small amount of hydrazine blended in nitromethane can increase the power output even further.
The National Hot Rod Association and Academy of Model Aeronautics do not permit its use in competitions.
It can be purified by cooling below its freezing point, washing the solid with cold diethyl ether, followed by distillation.
[28] Nitromethane was not known to be a high explosive until a railroad tank car loaded with it exploded on June 1, 1958.
It was thought that an operator rapidly snapped shut a valve creating a "hammer-lock" pressure surge.
[citation needed] If mixed with ammonium nitrate, which is used as an oxidizer, it forms an explosive mixture known as ANNM.
It has several advantages as a model explosive over TNT, namely its uniform density and lack of solid post-detonation species that complicate the determination of equation of state and further calculations.
This substance is a sensitive explosive which reverts to nitromethane under acidic conditions and decomposes in water to form another explosive compound, sodium methazonate, which has a reddish-brown color: Nitromethane's reaction with solid sodium hydroxide is hypergolic.