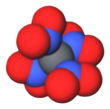
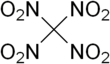Tetranitromethane
Highly purified tetranitromethane cannot be made to explode, but its sensitivity is increased dramatically by oxidizable contaminants, such as anti-freezing additives.
[7] TNM is a pale yellow liquid that can be prepared in the laboratory by the nitration of acetic anhydride with anhydrous nitric acid (Chattaway's method).
[8] This method was attempted on an industrial scale in the 1950s by Nitroform Products Company in Newark, USA, but the entire plant was destroyed by an explosion in 1953.
[9] The first industrial scale production was started in Germany during World War II in an effort to improve the cetane number of diesel fuel.
[12] First, nitric acid containing mercuric nitrate is reduced by acetylene, resulting in trinitromethane (nitroform) and a mixture of carbon dioxide and nitrogen oxide as waste gas.
Reduction of symmetry and analysis of the twinning of the crystals led finally to a resolved disorder of the structure shown in Figure 2.
A tragic lecture experiment at the University of Münster in 1920 is well known, where a small steel tube containing tetranitromethane, toluene and absorbent cotton detonated shortly before burning out in such a way that more than 30 students were injured, some seriously;[16] however, on the basis of the rector's office records, as many as 10 deaths and more than a dozen injuries are documented.
Absorption of as little as 2.5 mg/kg can cause methemoglobinemia, pulmonary edema, and damage to liver, kidney, and central nervous system.