Noscapine
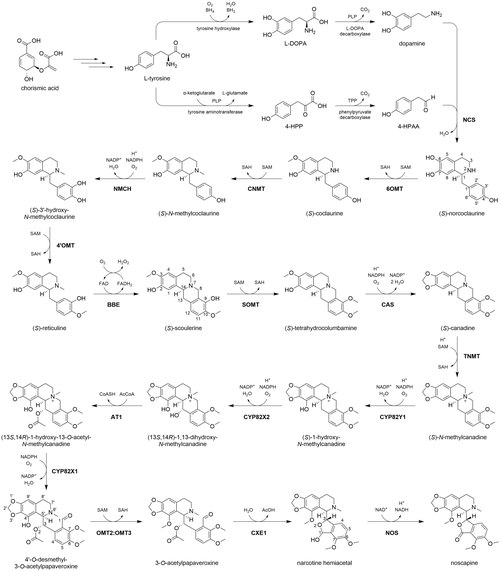
[5] The biosynthesis of noscapine in P. somniferum begins with chorismic acid, which is synthesized via the shikimate pathway from erythrose 4-phosphate and phosphoenolpyruvate.
Norcoclaurine synthase (NCS) catalyzes a Pictet-Spengler reaction between 4-HPAA and dopamine to synthesize (S)-norcoclaurine, providing the characteristic benzylisoquinoline scaffold.
Another cytochrome P450 enzyme (CYP82X1) catalyzes the hydroxylation of C8, and the newly formed hemiaminal spontaneously cleaves, yielding a tertiary amine and aldehyde.
[9] The O-acetyl group is then cleaved by a carboxylesterase (CXE1), yielding an alcohol which immediately reacts with the neighboring C1 aldehyde to form a hemiacetal in a new five-membered ring.
The apparent counteractivity between AT1 and CXE1 suggests that acetylation in this context is employed as a protective group, preventing hemiacetal formation until the ester is enzymatically cleaved.
[11] Noscapine, and its synthetic derivatives called noscapinoids, are known to interact with microtubules and inhibit cancer cell proliferation [12] The lactone ring is unstable and opens in basic media.
Noscapine was first isolated and characterized in chemical breakdown and properties in 1803 under the denomination of "Narcotine"[13][14] by Jean-Francois Derosne, a French chemist in Paris.
[20][21] Polyploidy induction by noscapine has been observed in vitro in human lymphocytes at high dose levels (>30 μM); however, low-level systemic exposure, e.g. with cough medications, does not appear to present a genotoxic hazard.
The mechanism of polyploidy induction by noscapine is suggested to involve either chromosome spindle apparatus damage or cell fusion.
[7] In 2016, the biosynthetic pathway of noscapine was reconstituted in yeast cells,[24] allowing the drug to be synthesised without the requirement of harvest and purification from plant material.
[25] It is hoped that this technology could be used to produce pharmaceutical alkaloids such as noscapine which are currently expressed at too low a yield in plantae to be mass-produced, allowing them to become marketable therapeutic drugs.
Moreover, they showed that the amount of inflammation reduction at this dose of noscapine is approximately equal to indomethacin, a standard anti-inflammatory medication.
Furthermore, Shiri et al. concluded that noscapine prevented the progression of bradykinin-induced inflammation in the rat's foot by antagonising bradykinin receptors.
Again, this brominated derivative also inhibits toll-like receptors (TLR), TNF-α, and nitric oxide (NO) in human and mouse macrophages without causing toxicity.