Nuclear reprocessing
In the United States, the Obama administration stepped back from President Bush's plans for commercial-scale reprocessing and reverted to a program focused on reprocessing-related scientific research.
[4] Not all nuclear fuel requires reprocessing; a breeder reactor is not restricted to using recycled plutonium and uranium.
It can employ all the actinides, closing the nuclear fuel cycle and potentially multiplying the energy extracted from natural uranium by about 60 times.
The only reprocessing required, therefore, was the extraction of the plutonium (free of fission-product contamination) from the spent natural uranium fuel.
The key issue driving this policy was the risk of nuclear weapons proliferation by diversion of plutonium from the civilian fuel cycle, and to encourage other nations to follow the US lead.
[14] In 2011 the New York Times reported "...11 years after the government awarded a construction contract, the cost of the project has soared to nearly $5 billion.
The vast concrete and steel structure is a half-finished hulk, and the government has yet to find a single customer, despite offers of lucrative subsidies."
TVA (currently the most likely customer) said in April 2011 that it would delay a decision until it could see how MOX fuel performed in the nuclear accident at Fukushima Daiichi.
Nevertheless, highly reliable nuclear weapons can be built at all levels of technical sophistication using reactor-grade plutonium.
[17] Many of these concepts, particularly those that separate the minor actinides once U and Pu have been extracted, are summarised in the advanced reprocessing of spent nuclear fuel.
The DIAMEX (DIAMide EXtraction) process has the advantage of avoiding the formation of organic waste which contains elements other than carbon, hydrogen, nitrogen, and oxygen.
[28] Other methods for the extraction of uranium using ion exchange in alkaline carbonate and "fumed" lead oxide have also been reported.
The bismuth phosphate process was designed to extract plutonium from aluminium-clad nuclear fuel rods, containing uranium.
The bismuth phosphate was next re-precipitated, leaving the plutonium in solution, and an iron(II) salt (such as ferrous sulfate) was added.
[33] The sodium uranyl acetate process was used by the early Soviet nuclear industry to recover plutonium from irradiated fuel.
[41][42][43][44] The electrolysis methods are based on the difference in the standard potentials of uranium, plutonium and minor actinides in a molten salt.
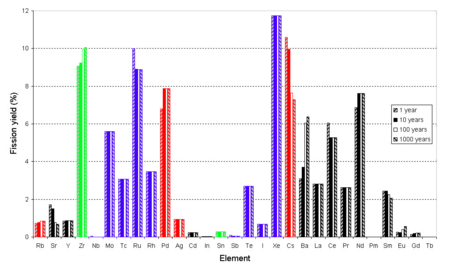
The radioactivity of this waste will then drop to the level of various naturally occurring minerals and ores within a few hundred, rather than thousands of, years.
[54] The mixed actinides produced by pyrometallic processing can be used again as nuclear fuel, as they are virtually all either fissile, or fertile, though many of these materials would require a fast breeder reactor to be burned efficiently.
In a thermal neutron spectrum, the concentrations of several heavy actinides (curium-242 and plutonium-240) can become quite high, creating fuel that is substantially different from the usual uranium or mixed uranium-plutonium oxides (MOX) that most current reactors were designed to use.
A typical transmuter fuel is free from uranium and contains recovered transuranics in an inert matrix such as metallic zirconium.
Newly generated technetium and iodine are extracted for incorporation into transmutation targets, and the other fission products are sent to waste.
Voloxidation also breaks up the fuel or increases its surface area to enhance penetration of reagents in following reprocessing steps.
Simply heating spent oxide fuel in an inert atmosphere or vacuum at a temperature between 700 °C (1,292 °F) and 1,000 °C (1,830 °F) as a first reprocessing step can remove several volatile elements, including caesium whose isotope caesium-137 emits about half of the heat produced by the spent fuel over the following 100 years of cooling (however, most of the other half is from strontium-90, which has a similar half-life).
Some transuranium elements such as plutonium, neptunium and americium can form volatile fluorides, but these compounds are not stable when the fluorine partial pressure is decreased.
Molten salt reactor designs carry out fluoride volatility reprocessing continuously or at frequent intervals.
Chlorides of alkaline elements like americium, curium, lanthanides, strontium, caesium are more soluble than those of uranium, neptunium, plutonium, and zirconium.
To determine the distribution of radioactive metals for analytical purposes, Solvent Impregnated Resins (SIRs) can be used.
The range of results produced by these studies is very wide, but all agreed that under then-current economic conditions the reprocessing-recycle option is the more costly one.
At the end of the year 2011 it became clear that Masaya Yasui, who had been director of the Nuclear Power Policy Planning Division in 2004, had instructed his subordinate in April 2004 to conceal the data.
The fact that the data were deliberately concealed obliged the ministry to re-investigate the case and to reconsider whether to punish the officials involved.