Nuclear transmutation
[1] Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
A transmutation can be achieved either by nuclear reactions (in which an outside particle reacts with a nucleus) or by radioactive decay, where no outside cause is needed.
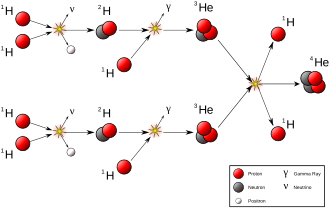
Natural transmutation by stellar nucleosynthesis in the past created most of the heavier chemical elements in the known existing universe, and continues to take place to this day, creating the vast majority of the most common elements in the universe, including helium, oxygen and carbon.
As stars begin to fuse heavier elements, substantially less energy is released from each fusion reaction.
Artificial nuclear transmutation has been considered as a possible mechanism for reducing the volume and hazard of radioactive waste.
[3] While alchemists often understood chrysopoeia as a metaphor for a mystical or religious process, some practitioners adopted a literal interpretation and tried to make gold through physical experimentation.
Alchemists like Michael Maier and Heinrich Khunrath wrote tracts exposing fraudulent claims of gold making.
It was first consciously applied to modern physics by Frederick Soddy when he, along with Ernest Rutherford in 1901, discovered that radioactive thorium was converting itself into radium.
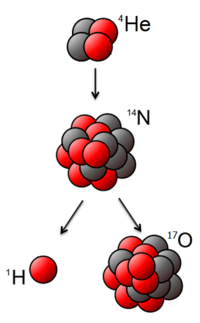
The first artificial transmutation was accomplished in 1925 by Patrick Blackett, a research fellow working under Rutherford, with the transmutation of nitrogen into oxygen, using alpha particles directed at nitrogen 14N + α → 17O + p.[7] Rutherford had shown in 1919 that a proton (he called it a hydrogen atom) was emitted from alpha bombardment experiments but he had no information about the residual nucleus.
The feat was popularly known as "splitting the atom", although it was not the modern nuclear fission reaction discovered in 1938 by Otto Hahn, Lise Meitner and their assistant Fritz Strassmann in heavy elements.
[8] In 1941, Rubby Sherr, Kenneth Bainbridge and Herbert Lawrence Anderson reported the nuclear transmutation of mercury into gold.
[citation needed] Glenn Seaborg succeeded in producing a minuscule amount of gold from bismuth, at a net energy loss.
More lithium, beryllium and boron were produced later, in a natural nuclear reaction, cosmic ray spallation.
Stellar nucleosynthesis is responsible for all of the other elements occurring naturally in the universe as stable isotopes and primordial nuclide, from carbon to uranium.
These are a type of red giant that "puffs" off its outer atmosphere, containing some elements from carbon to nickel and iron.
The Solar System is thought to have condensed approximately 4.6 billion years before the present, from a cloud of hydrogen and helium containing heavier elements in dust grains formed previously by a large number of such stars.
For example, the observed light curves of supernova stars such as SN 1987A show them blasting large amounts (comparable to the mass of Earth) of radioactive nickel and cobalt into space.
Transmutation of transuranium elements (i.e. actinides minus actinium to uranium) such as the isotopes of plutonium (about 1wt% in the light water reactors' used nuclear fuel or the minor actinides (MAs, i.e. neptunium, americium, and curium), about 0.1wt% each in light water reactors' used nuclear fuel) has the potential to help solve some problems posed by the management of radioactive waste by reducing the proportion of long-lived isotopes it contains.
Ceramic targets containing actinides can be bombarded with neutrons to induce transmutation reactions to remove the most difficult long-lived species.
The role of non-radioactive inert phases is mainly to provide stable mechanical behaviour to the target under neutron irradiation.
Of the remaining five long-lived fission products, selenium-79, tin-126 and palladium-107 are produced only in small quantities (at least in today's thermal neutron, 235U-burning light water reactors) and the last two should be relatively inert.