Deuterium
This figure is the highest yet measured in a comet, thus deuterium ratios continue to be an active topic of research in both astronomy and climatology.
Deuterium is thought to have played an important role in setting the number and ratios of the elements that were formed in the Big Bang.
Combining thermodynamics and the changes brought about by cosmic expansion, one can calculate the fraction of protons and neutrons based on the temperature at the point that the universe cooled enough to allow formation of nuclei.
At this point, the elemental abundances were nearly fixed, with the only change as some of the radioactive products of Big Bang nucleosynthesis (such as tritium) decay.
At the same time, the failure of much nucleogenesis during the Big Bang ensured that there would be plenty of hydrogen in the later universe available to form long-lived stars, such as the Sun.
The existence of deuterium on Earth, elsewhere in the Solar System (as confirmed by planetary probes), and in the spectra of stars, is also an important datum in cosmology.
Deuterium is produced by the rare cluster decay, and occasional absorption of naturally occurring neutrons by light hydrogen, but these are trivial sources.
The existence of deuterium at a low but constant primordial fraction in all hydrogen is another one of the arguments in favor of the Big Bang over the Steady State theory of the Universe.
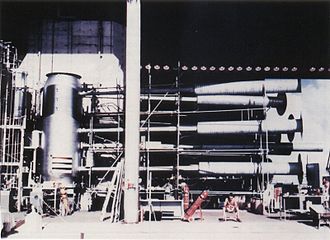
The world's leading supplier of deuterium was Atomic Energy of Canada Limited until 1997, when the last heavy water plant was shut down.
Infrared spectroscopy also easily differentiates many deuterated compounds, due to the large difference in IR absorption frequency seen in the vibration of a chemical bond containing deuterium, versus light hydrogen.
Deuterium, however, benefits from having its proton and neutron coupled to a spin-1 state, which gives a stronger nuclear attraction; the corresponding spin-1 state does not exist in the two-neutron or two-proton system, due to the Pauli exclusion principle which would require one or the other identical particle with the same spin to have some other different quantum number, such as orbital angular momentum.
[29] Due to the similarity in mass and nuclear properties between the proton and neutron, they are sometimes considered as two symmetric types of the same object, a nucleon.
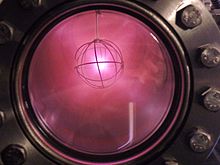
In research reactors, liquid 2H2 is used in cold sources to moderate neutrons to very low energies and wavelengths appropriate for scattering experiments.
Nuclear magnetic resonance spectroscopy can also be used to obtain information about the deuteron's environment in isotopically labelled samples (deuterium NMR).
[31] Deuterium NMR spectra are especially informative in the solid state because of its relatively small quadrupole moment in comparison with those of bigger quadrupolar nuclei such as chlorine-35, for example.
[32][33] In chemistry, biochemistry and environmental sciences, deuterium is used as a non-radioactive, stable isotopic tracer, for example, in the doubly labeled water test.
Measurements of small variations in the natural abundances of deuterium, along with those of the stable heavy oxygen isotopes 17O and 18O, are of importance in hydrology, to trace the geographic origin of Earth's waters.
Stable isotopes are incorporated into plants and animals and an analysis of the ratios in a migrant bird or insect can help suggest a rough guide to their origins.
[35][36] Neutron scattering techniques particularly profit from availability of deuterated samples: The 1H and 2H cross sections are very distinct and different in sign, which allows contrast variation in such experiments.
Hydrogen is an important and major component in all materials of organic chemistry and life science, but it barely interacts with X-rays.
[46] Deuterium has been shown to lengthen the period of oscillation of the circadian clock when dosed in rats, hamsters, and Gonyaulax dinoflagellates.
[49] Experiments in hamsters also support the theory that deuterium acts directly on the suprachiasmatic nucleus to lengthen the free-running circadian period.
[citation needed] At that time the neutron had not yet been discovered, and the prevailing theory was that isotopes of an element differ by the existence of additional protons in the nucleus accompanied by an equal number of nuclear electrons.
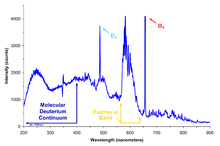
The cryogenic boiloff technique concentrated the fraction of the mass-2 isotope of hydrogen to a degree that made its spectroscopic identification unambiguous.
[55][56][57][4] Shortly before the war, Hans von Halban and Lew Kowarski moved their research on neutron moderation from France to Britain, smuggling the entire global supply of heavy water (which had been made in Norway) across in twenty-six steel drums.
[58][59] During World War II, Nazi Germany was known to be conducting experiments using heavy water as moderator for a nuclear reactor design.
Ultimately it led to the Allied operation called the "Norwegian heavy water sabotage", the purpose of which was to destroy the Vemork deuterium production/enrichment facility in Norway.
After World War II ended, the Allies discovered that Germany was not putting as much serious effort into the program as had been previously thought.
The Germans had completed only a small, partly built experimental reactor (which had been hidden away) and had been unable to sustain a chain reaction.
Released information suggests that all thermonuclear weapons built since then contain chemical compounds of deuterium and lithium in their secondary stages.