Proton-transfer-reaction mass spectrometry
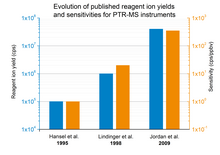
[1] PTR-MS is used for online monitoring of volatile organic compounds (VOCs) in ambient air and was developed in 1995 by scientists at the Institut für Ionenphysik at the Leopold-Franzens University in Innsbruck, Austria.
Commercially available PTR-MS instruments have a response time of about 100 ms and reach a detection limit in the single digit pptv or even ppqv region.
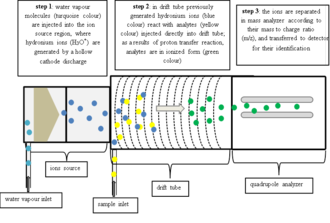
In commercial PTR-MS instruments water vapor is ionized in a hollow cathode discharge: After the discharge a short drift tube is used to form very pure (>99.5%[4]) H3O+ via ion-molecule reactions: Due to the high purity of the reagent ions a mass filter between the ion source and the reaction drift tube is not necessary and H3O+ can be injected directly.
The absence of this mass filter in turn greatly reduces losses of reagent ions and leads eventually to an outstandingly low detection limit of the whole instrument.
The main advantage of using NH4+ reagent ions is that fragmentation of analytes upon chemical ionization is strongly suppressed, leading to straightforward mass spectra even for complex mixtures.
The reason why during the first 20 years after the invention of PTR-MS NH4+ reagent ions have only been used in a very limited number of studies is most probably because the NH4+ production required toxic and corrosive ammonia as a source gas.
[7] In this method N2 and water vapor are introduced into the hollow cathode ion source and by adjusting electric fields and pressures NH4+ can be produced at the same or even higher purity levels than H3O+.
[8] Advantages include low fragmentation – only a small amount of energy is transferred during the ionization process (compared to e.g. electron ionization), therefore fragmentation is suppressed and the obtained mass spectra are easily interpretable, no sample preparation is necessary – VOC containing air and liquids' headspaces can be analyzed directly, real-time measurements – with a typical response time of 100 ms VOCs can be monitored on-line, real-time quantification – absolute concentrations are obtained directly without previous calibration measurements, compact and robust setup – due to the simple design and the low number of parts needed for a PTR-MS instrument, it can be built in into space saving and even mobile housings, easy to operate – for the operation of a PTR-MS only electric power and a small amount of distilled water are needed.
[9] In 2012 a PTR-MS instrument was introduced which extends the selectable reagent ions to Kr+ and Xe+;[10] this should allow for the detection of nearly all possible substances (up to the ionization energy of krypton (14 eV[11])).
Equation (2) is based on the assumption that the decrease of reagent ions is negligible, therefore the total concentration of VOCs in air must not exceed about 10 ppmv.
However, until 2012 these improvements were limited to optimizations of the conventional setup, i.e. ion source, DC drift tube, transfer lens system, mass spectrometer (compare above).
[13] That is, because of the highly compound dependent instrumental response one of the main advantages of PTR-MS, namely that concentration values can be directly calculated, is lost and a calibration measurement is needed for each analyte of interest.
Furthermore, with this approach unusual fragmentation of analytes has been observed[14] which complicates interpretation of measurement results and comparison between different types of instruments even more.
In 2014 Sulzer et al.[17] published an article about a PTR-MS instrument which utilizes a quadrupole ion guide between the drift tube and the TOF mass spectrometer.
Gas chromatography (GC) in combination with mass spectrometry (GC-MS) is capable of separating isomeric compounds.
Materic et al.[21] utilized an early version of a commercially available fastGC addon in order to distinguish various monoterpene isomers.
[23] The add-on consists of a honeycomb activated charcoal denuder which adsorbs organic gases but transmits particles, an aerodynamic lens system that collimates sub-μm particles, and a thermo-desorber that evaporates non-refractory organic particulate matter at moderate temperatures of 100-160 °C and reduced pressures of a few mbar.
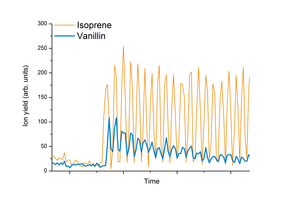
The test person swallows a sip of a vanillin flavored drink and breathes via his nose into a heated inlet device coupled to a PTR-MS instrument.
[43][44] Lindinger et al. developed a method to convert "dry" data from a PTR-MS instrument that measured headspace air from different coffee samples into expressions of flavor (e.g. "woody", "winey", "flowery", etc.)
and showed that the obtained flavor profiles matched nicely to the ones created by a panel of European coffee tasting experts.
3 a mass spectrum of air inside a laboratory (obtained with a time-of-flight (TOF) based PTR-MS instrument), is shown.
3 are in fact double, triple or multiple peaks (isobaric compounds) it becomes obvious that for PTR-MS instruments selectivity is at least as important as sensitivity, especially when complex samples / compositions are analyzed.
With the additional information obtained by using different reagent ions a much higher level of selectivity can be reached, e.g. some isomeric molecules can be distinguished.