PatientsLikeMe
PatientsLikeMe was inspired by the life experiences of Stephen Heywood, diagnosed in 1998 at the age of 29 with amyotrophic lateral sclerosis (ALS), or Lou Gehrig's disease.
[4] In 2017, PatientsLikeMe entered into a partnership with iCarbonX to apply next-generation biological measures and machine learning to understand more about the basis of human health and disease.
iCarbonX, founded in 2015 by renowned genomicist Jun Wang, took an equity position in PatientsLikeMe and provided multi-omics characterization services to the company.
[5] Unitedhealth Group and PatientsLikeMe made plans to help patients with similar health concerns connect to share experiences.
[7] From there, the company began adding other communities for other life-changing conditions, including multiple sclerosis (MS), Parkinson's disease, fibromyalgia, HIV, chronic fatigue syndrome, mood disorders, epilepsy,[8] organ transplantation, progressive supranuclear palsy, multiple system atrophy, and Devic's disease (neuromyelitis optica).
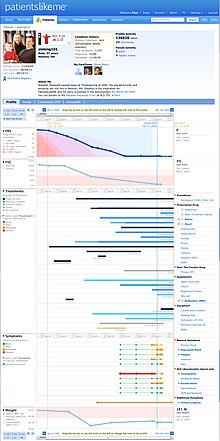
[14] PatientsLikeMe allows members to input real-world data on their conditions, treatment history, side effects, hospitalizations, symptoms, disease-specific functional scores, weight, mood, quality of life, and more on an ongoing basis.
The result is a detailed longitudinal record – organized into charts and graphs – that allows patients to gain insight and identify patterns.
Some communities, such as ALS, feature visual aids such as percentile curves on the patient profile, so that an individual user can see whether their rate of progression is fast, slow, or about average.
A seizure tracker for patients with epilepsy helps identify triggers such as missed medication doses, sleep deprivation, or alcohol use,[16] and a "mood map" for patients with mood disorders helps to show different factors underlying their condition such as emotional control, anxiety, or external stress while all users can look for patterns in their daily health status such as day of the week or time of day.
The PLM database includes information such as symptom reports from ALS patients that add data to the clinical trial.
[24] The site makes revenue by conducting scientific research studies for pharmaceutical companies, typically with an emphasis on issues that are important to both patients and industry.
[27] A 2016 collaboration with Novartis published in Nature Biotechnology and Value in Health explored ways in which patients could provide systematic input into guiding drug development to help make it more patient-centered.
Following the award in 2013 [31] and 2014 [32] of $4.5m in grants from the Robert Wood Johnson Foundation, the company developed an online tool called the Open Research Exchange (ORE) that allowed for the rapid creation, prototyping, testing, and validation of patient reported outcome measures, questionnaires that can establish the impact of symptoms and disease on patients.
[38] This has permitted PatientsLikeMe's research team to author more than 100 peer-reviewed published scientific articles in collaboration with academic and commercial partners in leading journals such as the BMJ, Nature Biotechnology, and Neurology.
[46] Using the self-reported data of 348 ALS patients, PatientsLikeMe conducted a 9-month long study which demonstrated that lithium did not slow the progress of the disease.
[47] The team suggested that online collection of patient self-report data was not a substitute for randomized placebo-controlled trials, but it might be a useful new form of clinical research in certain circumstances.
A later study described how patients attempted to use the same tools to unblind clinical trials in which they were enrolled to try and see whether or not the experimental drugs they were taking were working.