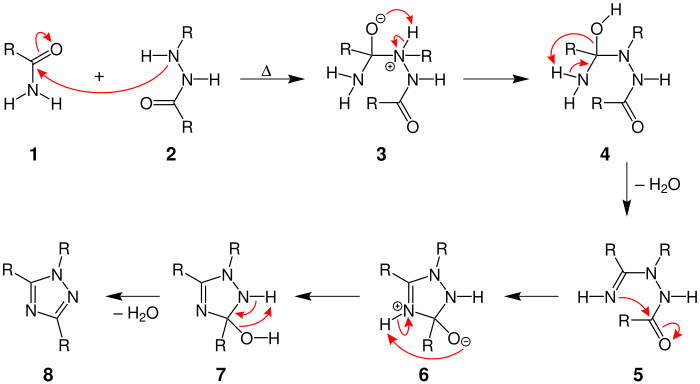
Pellizzari reaction
The mechanism begins by the nitrogen in the hydrazide attacking the carbonyl carbon on the amide to form compound 3.
The negatively charged oxygen then abstracts two hydrogens from neighboring nitrogens in order for a molecule of water to be released to form compound 5.
The nitrogen then performs an intramolecular attack on the carbonyl group to form the five-membered ring of compound 6.
[3] The Pellizzari reaction is limited in the number of substituents that can be on the ring, so other methods have been developed to incorporate three elements of diversity.
However, adding microwave irradiation shortens the reaction time and increases its yield.