Chromate and dichromate
In acid solution the unstable blue peroxo complex Chromium(VI) oxide peroxide, CrO(O2)2, is formed; it is an uncharged covalent molecule, which may be extracted into ether.
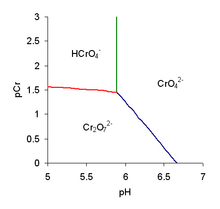
The predominance diagram shows that the position of the equilibrium depends on both pH and the analytical concentration of chromium.
The red line on the predominance diagram is not quite horizontal due to the simultaneous equilibrium with the chromate ion.
The hydrogen chromate ion may be protonated, with the formation of molecular chromic acid, H2CrO4, but the pKa for the equilibrium is not well characterized.
[8] Chromates and dichromates are used in chrome plating to protect metals from corrosion and to improve paint adhesion.
[7] When used as oxidizing agents or titrants in a redox chemical reaction, chromates and dichromates convert into trivalent chromium, Cr3+, salts of which typically have a distinctively different blue-green color.
Crocoite, PbCrO4, which can occur as spectacular long red crystals, is the most commonly found chromate mineral.
Also positive associations have been observed between exposure to chromium (VI) compounds and cancer of the nose and nasal sinuses.