Drug packaging
It involves all of the operations from production through drug distribution channels to the end consumer.
Pharmaceutical packaging is highly regulated but with some variation in the details, depending on the country of origin or the region.
Shipments to medical professionals could be at hospitals, nursing homes, veterinarians, dentists, etc.
These packaged pharmaceuticals are intended to be dispensed and administered by professionally trained and certified personnel.
Drugs under prescription control are sent to pharmacies in multi-packs of unit packs or in bottles containing many hundreds of capsules.
Typically a pharmacist prepares the final form of the unit pack or places a lower count of capsules in a small bottle for the customer.
Child resistant packaging is often required on the unit packs; if requested, a pharmacist is allowed put drugs in a bottle with easy open features.
Usually the packaging and labeling of dietary supplements, homeopathic drugs, and folk medicines are not regulated.
Some producers voluntarily follow the regulations for over-the-counter drugs or regional Pharmacopoeias.
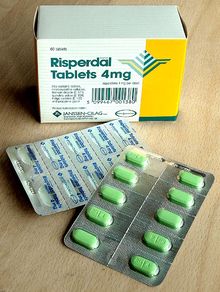
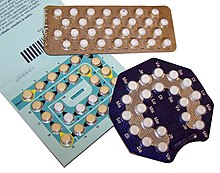
Some of the common primary packages are: Formed solid unit doses of pharmaceuticals (capsules, suppositories, tablets, etc.)
The primary component of a blister pack is a cavity or pocket made from a thermoformed plastic.
Blister packs are useful for protecting drugs against external factors, such as humidity and contamination for extended periods of time.
Other common colors include: Clear (for compounds that don't degrade in light), blue, dark brown, green, and various opaque hues.
Larger shipments are sent in insulated shipping containers with dry ice or gel packs.
Other methods of including desiccants attached to the inner surface or in the material have recently been developed.
[18] All aspects of pharmaceutical production, including packaging, are tightly controlled and have regulatory requirements.
Uniformity, cleanliness (washdown), sterility, and other requirements are needed to maintain Good Manufacturing Practices.