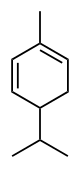
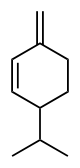
Phellandrene
In α-phellandrene, both double bonds are endocyclic, and in β-phellandrene, one of them is exocyclic.
α-Phellandrene undergoes hydrochlorination to give phellandrene hydrochloride (a cyclohexenyl chloride).
The resultant monoterpene undergoes cyclization to form a menthyl cationic species.
Finally, an elimination reaction occurs at one of two positions, yielding either α-phellandrene or β-phellandrene.
The α-phellandrene isomer can form hazardous and explosive peroxides on contact with air at elevated temperatures.