Boiling point
Because of this, water boils at 100°C (or with scientific precision: 99.97 °C (211.95 °F)) under standard pressure at sea level, but at 93.4 °C (200.1 °F) at 1,905 metres (6,250 ft)[3] altitude.
[6] The heat of vaporization is the energy required to transform a given quantity (a mol, kg, pound, etc.)
Liquids may change to a vapor at temperatures below their boiling points through the process of evaporation.
If the pressure in a system remains constant (isobaric), a vapor at saturation temperature will begin to condense into its liquid phase as thermal energy (heat) is removed.
Similarly, a liquid at saturation temperature and pressure will boil into its vapor phase as additional thermal energy is applied.
[6][8] For comparison, on top of Mount Everest, at 8,848 m (29,029 ft) elevation, the pressure is about 34 kPa (255 Torr)[9] and the boiling point of water is 71 °C (160 °F).
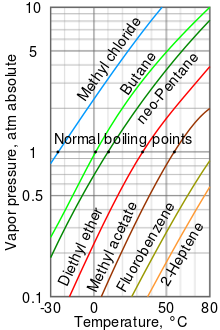
[10] As can be seen in the chart, the liquids with the highest vapor pressures have the lowest normal boiling points.
For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart.
If the compound's normal boiling point is higher, then that compound can exist as a liquid or solid at that given temperature at atmospheric external pressure, and will so exist in equilibrium with its vapor (if volatile) if its vapors are contained.
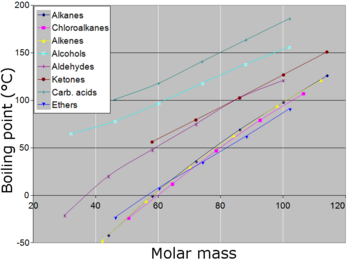
When the molecular size becomes that of a macromolecule, polymer, or otherwise very large, the compound often decomposes at high temperature before the boiling point is reached.
Closely related is the ability of a molecule to form hydrogen bonds (in the liquid state), which makes it harder for molecules to leave the liquid state and thus increases the normal boiling point of the compound.
By comparison to boiling, a sublimation is a physical transformation in which a solid turns directly into vapor, which happens in a few select cases such as with carbon dioxide at atmospheric pressure.
The presence of non-volatile impurities such as salts or compounds of a volatility far lower than the main component compound decreases its mole fraction and the solution's volatility, and thus raises the normal boiling point in proportion to the concentration of the solutes.
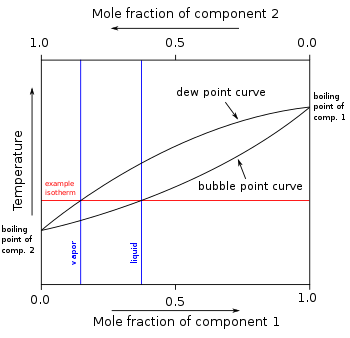
In order to illustrate these effects between the volatile components in a mixture, a boiling point diagram is commonly used.
Distillation is a process of boiling and [usually] condensation which takes advantage of these differences in composition between liquid and vapor phases.
Following is a table of the change in the boiling point of water with elevation, at intervals of 500 meters over the range of human habitation [the Dead Sea at −430.5 metres (−1,412 ft) to La Rinconada, Peru at 5,100 m (16,700 ft)], then of 1,000 meters over the additional range of uninhabited surface elevation [up to Mount Everest at 8,849 metres (29,032 ft)], along with a similar range in Imperial.