Phenacaine
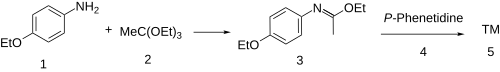
[2] The synthesis of phenacaine begins with the condensation of p-phenetidine (1) with triethyl orthoacetate (2) to afford the imino ether (a Pinner salt, 3).
Reaction of that intermediate with a second equivalent of the aniline results (4) in a net displacement of ethanol, probably by an addition-elimination scheme, producing the amidine, phenacaine (5).
In the patented synthesis,[4] phenacetin was used as precursor.
Treatment with phosphorus trichloride (PCl3) gave the enol chloride, and reaction of this intermediate with p-phenetidine then completed the synthesis of phenacaine.
This drug article relating to the nervous system is a stub.