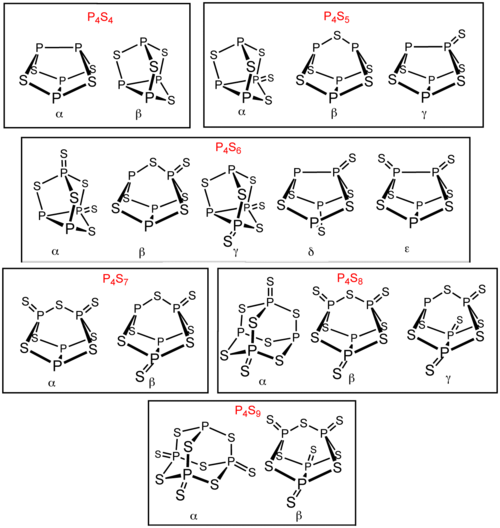
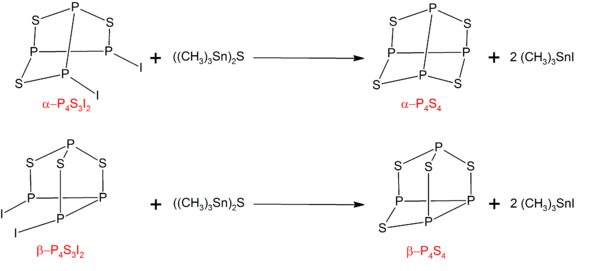Phosphorus sulfides
[3] Phosphorus monosulfide monomer, PS, is highly unstable and only exists at elevated temperatures.
An alternative method involves the controlled fusion of white phosphorus with sulfur in an inert, non-flammable solvent.
[8] The α- and β- forms of P4S4 can be prepared by treating the corresponding isomers of P4S3I2 with ((CH3)3Sn)2S:[7] P4S3I2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.
[11] P4S7 is most conveniently made by direct union of the corresponding elements, and is one of the most easily purified binary phosphorus sulfides.
[7] Another method involves the heating of P4S7 and P4S10 in 1:2 mole ratio, where P4S9 is reversibly formed:[11] P4S10 is one of the most stable phosphorus sulfides.