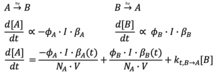Photoswitch
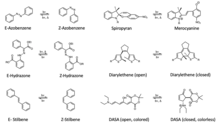
Several examples of photochromic compounds include: azobenzene,[6] spiropyran,[7] merocyanine,[8] diarylethene,[9] spirooxazine,[10] fulgide,[11] hydrazone,[12] nobormadiene,[13] thioindigo,[14] acrylamide-azobenzene-quaternary ammonia,[15] donor-acceptor Stenhouse adducts,[16][17] stilbene,[18] etc.
[23] Through chemical modification, red shifting the wavelengths of absorption needed to cause isomerizaiton leads to low light induced switching which has applications in photopharmacology.
[24] When a photochromic compound is incorporated into a suitable catalytic molecule, photoswitchable catalysis can result from the reversible changes in geometric conformation upon irradiation with light.
[25] As one of the most widely studied photoswitches, azobenzene has been shown to be an effective switch for regulating catalytic activity due to its isomerization from the E to Z conformation with light, and its ability to thermally relax back to the E isomer in dark conditions.
[26] One of the more prevalent biological examples in the human body that undergoes structural changes upon light irradiation includes the class of membrane-bound photoreceptors, Rhodopsins.
[29] Rhodopsins are highly efficient photochromic compounds that can undergo fast photoisomerization and are associated with various retinal proteins[30] along with light-gated channels and pumps in microbes.
Fast isomerization allows retinal cells to turn on when activated by light and advances in acrylamide-azobenzene-quaternary ammonia have shown restoration of visual responses in blind mice.
[39] Diarylethenes form stable molecular conduction junctions when placed between graphene electrodes at low and room temperature and act as a photo-electrical switch.
[40] By combining a photoswitch, containing various highest and lowest unoccupied molecular orbital levels in its open and closed geometrical conformation, into a film composed of either p- or n-doped semiconductors, charge transport can be controlled with light.
[44] Incorporation of photoswitching molecules such as donor-acceptor Stenhouse adducts into polymersomes has been used to form nanoparticles which can selectively expose enzymes in response to light, allowing them to mimic some functions of cells.
[53] Pharmaceutical encapsulation and distribution at targeted locations with light has been demonstrated due to the unique change in properties and size of microencapsulated nanostructures with photochromic components.