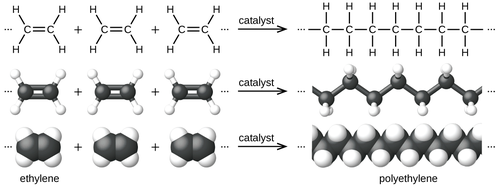
Polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic.
[8][9] Many kinds of polyethylene are known, with most having the chemical formula (C2H4)n. PE is usually a mixture of similar polymers of ethylene, with various values of n. It can be low-density or high-density and many variations thereof.
[12][a][13][b] When his colleagues Eugen Bamberger and Friedrich Tschirner characterized the white, waxy substance that he had created, they recognized that it contained long −CH2− chains and termed it polymethylene.
[15] Upon applying extremely high pressure (several hundred atmospheres) to a mixture of ethylene and benzaldehyde they again produced a white, waxy material.
Because polyethylene was found to have very low-loss properties at very high frequency radio waves, commercial distribution in Britain was suspended on the outbreak of World War II, secrecy imposed, and the new process was used to produce insulation for UHF and SHF coaxial cables of radar sets.
[16][17] The landmark breakthrough in the commercial production of polyethylene began with the development of catalysts that promoted the polymerization at mild temperatures and pressures.
[18] In 1953 the German chemist Karl Ziegler developed a catalytic system based on titanium halides and organoaluminium compounds that worked at even milder conditions than the Phillips catalyst.
It offers good electrical treeing resistance; however, it becomes easily electrostatically charged (which can be reduced by additions of graphite, carbon black or antistatic agents).
Depending on thermal history and film thickness, PE can vary between almost clear (transparent), milky-opaque (translucent) and opaque.
[22] The ingredient or monomer is ethylene (IUPAC name ethene), a gaseous hydrocarbon with the formula C2H4, which can be viewed as a pair of methylene groups (−CH2−) connected to each other.
Its mechanical properties depend significantly on variables such as the extent and type of branching, the crystal structure, and the molecular weight.
[25] The high molecular weight makes it a very tough material, but results in less efficient packing of the chains into the crystal structure as evidenced by densities of less than high-density polyethylene (for example, 0.930–0.935 g/cm3).
It is used in products and packaging such as milk jugs, detergent bottles, butter tubs, garbage containers, and water pipes.
PEX is a medium- to high-density polyethylene containing cross-link bonds introduced into the polymer structure, changing the thermoplastic into a thermoset.
PEX is used in some potable-water plumbing systems because tubes made of the material can be expanded to fit over a metal nipple and it will slowly return to its original shape, forming a permanent, water-tight connection.
LLDPE is a substantially linear polymer with significant numbers of short branches, commonly made by copolymerization of ethylene with short-chain alpha-olefins (for example, 1-butene, 1-hexene, and 1-octene).
Lower-thickness (gauge) films can be blown, compared with LDPE, with better environmental stress cracking resistance, but they are not as easy to process.
LDPE has a high degree of short- and long-chain branching, which means that the chains do not pack into the crystal structure as well.
[29] The Indian mealmoth larvae are claimed to metabolize polyethylene based on observing that plastic bags at a researcher's home had small holes in them.
Not only could the bacteria from the guts of the Plodia interpunctella moth larvae metabolize polyethylene, they degraded it significantly, dropping its tensile strength by 50%, its mass by 10% and the molecular weights of its polymeric chains by 13%.
The caterpillar is able to digest polyethylene due to a combination of its gut microbiota[35] and its saliva containing enzymes that oxidise and depolymerise the plastic.
[36] When exposed to ambient solar radiation the plastic produces trace amounts of two greenhouse gases, methane and ethylene.
Metallocene polyethylene has a relatively narrow molecular weight distribution, exceptionally high toughness, excellent optical properties and a uniform comonomer content.
The higher molecular weight fractions form linking molecules between crystallites, thereby increasing toughness and stress crack resistance.
Polyethylene with multimodal molecular weight distribution can be prepared either in two-stage reactors, by catalysts with two active centers on a carrier or by blending in extruders.
[19]: 238 Cyclic olefin copolymers are prepared by copolymerization of ethene and cycloolefins (usually norbornene) produced by using metallocene catalysts.
[19] If salts of an unsaturated carboxylic acid are present in the polymer, thermo-reversible ion networks are formed, they are called ionomers.
[39]: 235 Due to decreasing crystallinity ethylene vinyl acetate copolymers are getting softer with increasing comonomer content.
Braskem will build a new facility at their existing industrial unit in Triunfo, Rio Grande do Sul, Brazil with an annual production capacity of 200,000 short tons (180,000,000 kg), and will produce high-density and low-density polyethylene from bioethanol derived from sugarcane.
In the United Kingdom and India the polymer is commonly called polythene, from the ICI trade name, although this is not recognized scientifically.