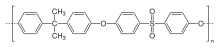
Polysulfone
Due to the high cost of raw materials and processing, polysulfones are used in specialty applications and often are a superior replacement for polycarbonates.
The simplest polysulfone poly(phenylene sulfone), known as early as 1960, is produced in a Friedel-Crafts reaction from benzenesulfonyl chloride:[3] With a melting point over 500 °C, the product is difficult to process.
The original synthesis of PAES involved electrophilic aromatic substitution of an diaryl ether with the bis (sulfonyl chloride) of benzene.
Reactions typically use a Friedel-Crafts catalyst, such as ferric chloride or antimony pentachloride: This route is complicated by the formation of isomers arising from both para- and ortho- substitution.
The polymerization is carried out at 130–160 °C under inert conditions in a polar, aprotic solvent, e.g. dimethyl sulfoxide, forming a polyether concomitant with elimination of sodium chloride:
[9] They have a high dimensional stability, the size change when exposed to boiling water or 150 °C air or steam generally falls below 0.1%.
For a comparison of the properties of individual constituents poly(phenylene sulfone) can serve as an example, which consists of sulfonyl and phenyl groups only.
However, the polymer chains are also so rigid that poly(phenylene sulfone) (PAS) decomposes before melting and can thus not be thermoplastically processed.
This leads to a significantly reduced melting point and also improves the mechanical properties by an increased impact strength.
As a result, larger amounts of energy from heat or radiation can be absorbed by the molecular structure without causing any reactions (decomposition).
Polysulfone allows easy manufacturing of membranes, with reproducible properties and controllable size of pores down to 40 nanometers.
Such membranes can be used in applications like hemodialysis, waste water recovery, food and beverage processing, and gas separation.
Filter cartridges made from polysulfone membranes offer extremely high flow rates at very low differential pressures when compared with nylon or polypropylene media.
Recently, sulfonated polyethersulfones (SPES) have been studied as a promising material candidate among many other aromatic hydrocarbon-based polymers for highly durable proton-exchange membranes in fuel cells.
Under oxidative environment, SPES can undergo sulfonic group detachment and main chain scission.
The wide working temperature range of -40°C to 190°C allow these pans to go from a deep freezer directly to a steam table or microwave oven.