Polyvinylidene fluoride
Its chemical formula is (C2H2F2)n. PVDF is a specialty plastic used in applications requiring the highest purity, as well as resistance to solvents, acids and hydrocarbons.
It can be injected, molded or welded and is commonly used in the chemical, semiconductor, medical and defense industries, as well as in lithium-ion batteries.
They are in use on many prominent buildings around the world, such as the Petronas Towers in Malaysia and Taipei 101 in Taiwan, as well as on commercial and residential metal roofing.
PVDF membranes are used in western blots for the immobilization of proteins, due to its non-specific affinity for amino acids.
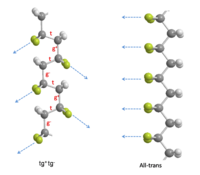
Unlike other popular piezoelectric materials, such as lead zirconate titanate (PZT), PVDF has a negative d33 value.
[8] Fluorinated polymers like PTFE and PVDF are especially thermally stable due to strong carbon-fluorine (C–F) bonds, the strongest in organic chemistry, which contribute to the durability of these materials under heat.
Above 316 °C, PVDF decomposes via dehydrofluorination, which can lead to structural changes, including double bonds and potential discoloration from thermal decomposition.
Namely these are: ozone oxidation reactions, nuclear radiation, UV damage, and microbiological, fungus growth.
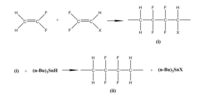
[citation needed] PVDF may be synthesized from the gaseous vinylidene fluoride (VDF) monomer by a free-radical (or controlled-radical) polymerization process.
In aqueous emulsion polymerization, the fluorosurfactant perfluorononanoic acid is used in anion form as a processing aid by solubilizing monomers.
Thick films (typically >100 μm) must be heated during the poling process in order to achieve a large piezoelectric response.
The piezoelectric properties of PVDF are exploited in the manufacture of tactile sensor arrays, inexpensive strain gauges, and lightweight audio transducers.
This slurry is cast onto a metallic current collector, and the NMP is evaporated to form a composite or paste electrode.
In the biomedical sciences, PVDF is used in immunoblotting as an artificial membrane (usually with 0.22 or 0.45-micrometre pore sizes), on which proteins are transferred using electricity (see western blotting).
The various properties of this material, such as heat resistance, resistance to chemical corrosion, and low protein binding properties, make this material valuable in the biomedical sciences for preparation of medications as a sterilizing filter, and as a filter to prepare samples for analytical techniques such as high-performance liquid chromatography (HPLC), where small amounts of particulate matter can damage sensitive and expensive equipment.
For those reasons, the use of PVDF active sensors is a keystone for the development of future structural-health monitoring methods, due to their low cost and compliance.
Examples of PVDF uses include nuclear reactor waste handling, chemical synthesis and production, (sulfuric acid, common), air plenums, and boiler service pipe.
This results in a larger piezoelectric response: d33 values for P(VDF-TFE) have been recorded to be as high as −38 p C/N[19] compared to −33 pC/N in pure PVDF.
This random incorporation of CTFE in P(VDF-TrFE) copolymer disrupts the long-range ordering of the ferroelectric polar phase, resulting in the formation of nano-polar domains.
[29] Proposed regulations in the EU aim to ban "any substance that contains at least one fully fluorinated methyl (CF3) or methylene (CF2-) carbon atom (without any H/Cl/Br/I attached to it)”.