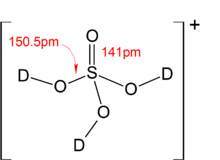
Sulfuric acid
[7] Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals.
It is most commonly used in fertilizer manufacture[11] but is also important in mineral processing, oil refining, wastewater treating, and chemical synthesis.
It has a wide range of end applications, including in domestic acidic drain cleaners,[12] as an electrolyte in lead-acid batteries, as a dehydrating compound, and in various cleaning agents.
Concentrated sulfuric acid has a powerful dehydrating property, removing water (H2O) from other chemical compounds such as table sugar (sucrose) and other carbohydrates, to produce carbon, steam, and heat.
[26][27] Sulfuric acid is rarely encountered naturally on Earth in anhydrous form, due to its great affinity for water.
When sulfur-containing fuels such as coal or oil are burned, sulfur dioxide is the main byproduct (besides the chief products carbon oxides and water).
[citation needed] Alternatively, dissolving sulfur dioxide in an aqueous solution of an oxidizing metal salt such as copper(II) or iron(III) chloride:[citation needed] Two less well-known laboratory methods of producing sulfuric acid, albeit in dilute form and requiring some extra effort in purification, rely on electrolysis.
The solution of dilute sulfuric acid indicates completion of the reaction when it turns from blue to clear (production of hydrogen at cathode is another sign):[citation needed] More costly, dangerous, and troublesome is the electrobromine method, which employs a mixture of sulfur, water, and hydrobromic acid as the electrolyte.
About 20% is used in chemical industry for production of detergents, synthetic resins, dyestuffs, pharmaceuticals, petroleum catalysts, insecticides and antifreeze, as well as in various processes such as oil well acidicizing, aluminium reduction, paper sizing, and water treatment.
About 6% of uses are related to pigments and include paints, enamels, printing inks, coated fabrics and paper, while the rest is dispersed into a multitude of applications such as production of explosives, cellophane, acetate and viscose textiles, lubricants, non-ferrous metals, and batteries.
Much H2SO4 is used in petroleum refining, for example as a catalyst for the reaction of isobutane with isobutylene to give isooctane, a compound that raises the octane rating of gasoline (petrol).
The study of vitriols (hydrated sulfates of various metals forming glassy minerals from which sulfuric acid can be derived) began in ancient times.
Metallurgical uses for vitriolic substances were recorded in the Hellenistic alchemical works of Zosimos of Panopolis, in the treatise Phisica et Mystica, and the Leyden papyrus X.
[40] In one recipe recorded in his Kitāb al-Asrār ('Book of Secrets'), al-Razi may have created sulfuric acid without being aware of it:[41] Take white (Yemeni) alum, dissolve it and purify it by filtration.
vitriol with copper-green (the acetate), and mix (the distillate) with the filtered solution of the purified alum, afterwards let it solidify (or crystallise) in the glass beaker.
[42]In an anonymous Latin work variously attributed to Aristotle (under the title Liber Aristotilis, 'Book of Aristotle'),[43] to al-Razi (under the title Lumen luminum magnum, 'Great Light of Lights'), or to Ibn Sina,[44] the author speaks of an 'oil' (oleum) obtained through the distillation of iron(II) sulfate (green vitriol), which was likely 'oil of vitriol' or sulfuric acid.
[49] According to Ahmad Y. al-Hassan, three recipes for sulfuric acid occur in an anonymous Garshuni manuscript containing a compilation taken from several authors and dating from before c. 1100 AD.
[51]A recipe for the preparation of sulfuric acid is mentioned in Risālat Jaʿfar al-Sādiq fī ʿilm al-ṣanʿa, an Arabic treatise falsely attributed to the Shi'i Imam Ja'far al-Sadiq (died 765).
In the 17th century, Johann Rudolf Glauber discovered that adding saltpeter (potassium nitrate, KNO3) significantly improves the output, also replacing moisture with steam.
In 1746 in Birmingham, John Roebuck adapted this method to produce sulfuric acid in lead-lined chambers, which were stronger, less expensive, and could be made larger than the previously used glass containers.
[33] In the early to mid 19th century "vitriol" plants existed, among other places, in Prestonpans in Scotland, Shropshire and the Lagan Valley in County Antrim, Northern Ireland, where it was used as a bleach for linen.
In common with other corrosive acids and alkali, it readily decomposes proteins and lipids through amide and ester hydrolysis upon contact with living tissues, such as skin and flesh.
In addition, it exhibits a strong dehydrating property on carbohydrates, liberating extra heat and causing secondary thermal burns.
The standard first aid treatment for acid spills on the skin is, as for other corrosive agents, irrigation with large quantities of water.
Washing is continued for at least ten to fifteen minutes to cool the tissue surrounding the acid burn and to prevent secondary damage.
Heat generated in this thin layer of water can boil, leading to the dispersal of a sulfuric acid aerosol, or worse, an explosion.
Preparation of solutions greater than 6 M (35%) in concentration is dangerous, unless the acid is added slowly enough to allow the mixture sufficient time to cool.
The main occupational risks posed by this acid are skin contact leading to burns (see above) and the inhalation of aerosols.
Repeated occupational exposure to sulfuric acid mists may increase the chance of lung cancer by up to 64 percent.
International commerce of sulfuric acid is controlled under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances, 1988, which lists sulfuric acid under Table II of the convention as a chemical frequently used in the illicit manufacture of narcotic drugs or psychotropic substances.