Propane
A by-product of natural gas processing and petroleum refining, it is often a constituent of liquefied petroleum gas (LPG), which is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation; other constituents of LPG may include propylene, butane, butylene, butadiene, and isobutylene.
[7] LPG powers buses, forklifts, automobiles, outboard boat motors, and ice resurfacing machines, and is used for heat and cooking in recreational vehicles and campers.
[12][13] Walter O. Snelling of the U.S. Bureau of Mines highlighted it as a volatile component in gasoline in 1910, which marked the "birth of the propane industry" in the United States.
[15] It was during this time that Snelling—in cooperation with Frank P. Peterson, Chester Kerr, and Arthur Kerr—developed ways to liquefy the LP gases during the refining of gasoline.
[14] A separate method of producing LP gas through compression was developed by Frank Peterson and its patent was granted on July 2, 1912.
Major industry developments in the 1930s included the introduction of railroad tank car transport, gas odorization, and the construction of local bottle-filling plants.
[14] In 1950, 1,000 propane-fueled buses were ordered by the Chicago Transit Authority, and by 1958, sales in the U.S. had reached 7 billion US gallons (26,000,000 m3) annually.
Ethyl mercaptan is added as a safety precaution as an odorant,[23] and is commonly called a "rotten egg" smell.
[28] Propane burns hotter than home heating oil or diesel fuel because of the very high hydrogen content.
[27] The enthalpy of combustion of propane gas where products do not return to standard state, for example where the hot gases including water vapor exit a chimney, (known as lower heating value) is −2043.455 kJ/mol.
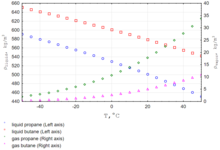
[30] As the density of propane changes with temperature, this fact must be considered every time when the application is connected with safety or custody transfer operations.
[31] Propane is a popular choice for barbecues and portable stoves because the low boiling point of −42 °C (−44 °F) makes it vaporize as soon as it is released from its pressurized container.
[32] Compared to fluorocarbons, propane has a negligible ozone depletion potential and very low global warming potential (having a GWP value of 0.072,[33] 13.9 times lower than the GWP of carbon dioxide) and can serve as a functional replacement for R-12, R-22, R-134a, and other chlorofluorocarbon or hydrofluorocarbon refrigerants in conventional stationary refrigeration and air conditioning systems.
[39] Since it can be transported easily, it is a popular fuel for home heat and backup electrical generation in sparsely populated areas that do not have natural gas pipelines.
The research also shows that gas and propane fuels appear to be the dominant source of benzene produced by cooking.
[40] In rural areas of North America, as well as northern Australia, propane is used to heat livestock facilities, in grain dryers, and other heat-producing appliances.
Large tractor-trailer trucks, with an average cylinder size of 10,000 US gallons (38 m3), transport propane from the pipeline or refinery to the local bulk plant.
The bobtail tank truck is not unique to the North American market, though the practice is not as common elsewhere, and the vehicles are generally called tankers.
This allows fast refill times, affordable fuel cylinder construction, and price ranges typically just over half that of gasoline.
there have been lawn-care products like string trimmers, lawn mowers and leaf blowers intended for outdoor use, but fueled by propane in order to reduce air pollution.
[44] Many heavy-duty highway trucks use propane as a boost, where it is added through the turbocharger, to mix with diesel fuel droplets.
Propane droplets' very high hydrogen content helps the diesel fuel to burn hotter and therefore more completely.
It is normal for a 7-liter medium-duty diesel truck engine to increase fuel economy by 20 to 33 percent when a propane boost system is used.
Typically in the United States and Canada, LPG is primarily propane (at least 90%), while the rest is mostly ethane, propylene, butane, and odorants including ethyl mercaptan.
"The exact proportion of this combination varies by country, depending on international prices, on the availability of components and, especially, on the climatic conditions that favor LPG with higher butane content in warmer regions and propane in cold areas".
If a leak in a propane fuel system occurs, the vaporized gas will have a tendency to sink into any enclosed area and thus poses a risk of explosion and fire.
In 2007, a heavily investigated vapor-related explosion occurred in Ghent, West Virginia, U.S., killing four people and completely destroying the Little General convenience store on Flat Top Road, causing several injuries.
[54][55] Another hazard associated with propane storage and transport is known as a BLEVE or boiling liquid expanding vapor explosion.
[41] The United States imports about 10% of the propane consumed each year, with about 70% of that coming from Canada via pipeline and rail.
As of October 2013[update], the retail cost of propane was approximately $2.37 per gallon, or roughly $25.95 per 1 million BTUs.