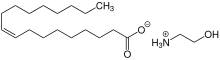
Protic ionic liquid
[1] Because the proton transfer reaction is reversible, the equilibrium between reactants and ionic products can shift depending on the conditions.
This equilibration significantly impact the properties of protic ionic liquids since some neutral acid and base species are generally present in the solution.
[1][5][6] Additionally, during distillation protic ionic liquids exhibit what appears to be a reactive azeotrope.
[7] However, when mixed with water the vaporization behavior is different and an azeotrope no longer occurs at that same composition.
[9] The density of a protic ionic liquid mixture also appears to be higher for the composition at which the azeotrope forms.