1,1,1,2-Tetrafluoroethane
1,1,1,2-Tetrafluoroethane (also known as norflurane (INN), R-134a, Klea 134a, Freon 134a, Forane 134a, Genetron 134a, Green Gas, Florasol 134a, Suva 134a, HFA-134a, or HFC-134a) is a hydrofluorocarbon (HFC) and haloalkane refrigerant with thermodynamic properties similar to R-12 (dichlorodifluoromethane) but with insignificant ozone depletion potential and a lower 100-year global warming potential (1,430, compared to R-12's GWP of 10,900).
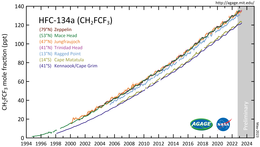
[10] 1,1,1,2-Tetrafluoroethane was introduced in the early 1990s as a replacement for dichlorodifluoromethane (R-12), which has massive ozone depleting properties.
[12] R-134a began being phased out from use in the European Union, starting in the mid 2010s, by a directive of 2006, recommending the replacement of gases in air conditioning systems with a GWP above 100.
The Society of Automotive Engineers (SAE) has proposed that it be best replaced by a new fluorochemical refrigerant HFO-1234yf (CF3CF=CH2) in automobile air-conditioning systems.
[14] As of model year 2021, newly manufactured light-duty vehicles in the United States no longer use R-134a.
[3] California may also prohibit the sale of canned R-134a to individuals to avoid non-professional recharge of air conditioners.
[15] A ban had been in place in Wisconsin since October 1994 under ATCP 136 prohibiting sales of container sizes holding less than 15 lbs of 1,1,1,2-tetrafluoroethane, but this restriction applied only when the chemical was intended to be a refrigerant.
However, mixtures with high concentrations of air at elevated pressure and/or temperature can be ignited.
Under pressure, 1,1,1,2-tetrafluoroethane is compressed into a liquid, which upon vaporization absorbs a significant amount of thermal energy.