Radioactivity in the life sciences
Radioactivity is generally used in life sciences for highly sensitive and direct measurements of biological phenomena, and for visualizing the location of biomolecules radiolabelled with a radioisotope.
In the case of the hydrogen isotope tritium (half-life = 12.3 years) and carbon-14 (half-life = 5,730 years), these isotopes derive their importance from all organic life containing hydrogen and carbon and therefore can be used to study countless living processes, reactions, and phenomena.
Radiolabeling is a technique used to track the passage of a molecule that incorporates a radioisotope through a reaction, metabolic pathway, cell, tissue, organism, or biological system.
Alternatively, molecules can be radiolabeled by chemical reactions that introduce an atom, moiety, or functional group that contains a radionuclide.
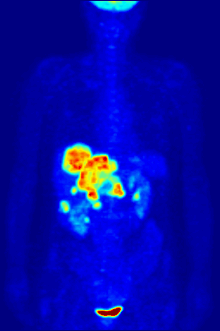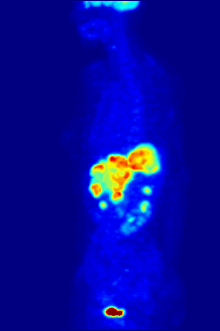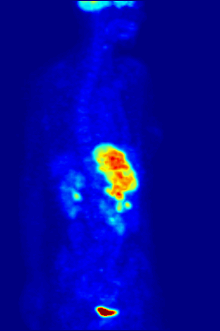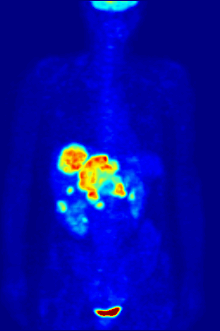
Again, a key feature of using radioactivity in life science applications is that it is a quantitative technique, so PET/SPECT not only reveals where a radiolabelled molecule is but how much is there.
Tritium (hydrogen-3) is a very low beta energy emitter that can be used to label proteins, nucleic acids, drugs and almost any organic biomolecule.
For tritium detection, liquid scintillation counters have been classically employed, in which the energy of a tritium decay is transferred to a scintillant molecule in solution which in turn gives off photons whose intensity and spectrum can be measured by a photomultiplier array.
Alternatively, a solid-state, tritium-specific phosphor screen can be used together with a phosphorimager to measure and simultaneously image the radiotracer.
[6] Carbon-14 labeling is common in drug development to do ADME (absorption, distribution, metabolism and excretion) studies in animal models and in human toxicology and clinical trials.
Quantitative Whole Body Autoradiography (QWBA): Larger than micro-autoradiography, whole animals, typically rodents, can be analyzed for biodistribution studies.
Not all molecules in the solution have a P-32 on the last (i.e., gamma) phosphate: the "specific activity" gives the radioactivity concentration and depends on the radionuclei's half-life.
The primary advantage of fluorescence over radiotracers is that it does not require radiological controls and their associated expenses and safety measures.
The decay of radioisotopes may limit the shelf life of a reagent, requiring its replacement and thus increasing expenses.
FRET), whereas with radioactivity two isotopes can be used (tritium and a low energy isotope, e.g. 33P due to different intensities) but require special equipment (a tritium screen and a regular phosphor-imaging screen, a specific dual channel detector, e.g. [1]).
If good health physics controls are maintained in a laboratory where radionuclides are used, it is unlikely that the overall radiation dose received by workers will be of much significance.
Nevertheless, the effects of low doses are mostly unknown so many regulations exist to avoid unnecessary risks, such as skin or internal exposure.
Due to the low penetration power and many variables involved it is hard to convert a radioactive concentration to a dose.
1 μCi of P-32 on a square centimetre of skin (through a dead layer of a thickness of 70 μm) gives 7961 rads (79.61 grays) per hour.