Radon
Theoretical studies on this molecule predict that it should have a Rn–F bond distance of 2.08 ångströms (Å), and that the compound is thermodynamically more stable and less volatile than its lighter counterpart xenon difluoride (XeF2).
[27] The [RnF]+ ion is believed to form by the following reaction:[28] For this reason, antimony pentafluoride together with chlorine trifluoride and N2F2Sb2F11 have been considered for radon gas removal in uranium mines due to the formation of radon–fluorine compounds.
Electromigration studies also suggest the presence of cationic [HRnO3]+ and anionic [HRnO4]− forms of radon in weakly acidic aqueous solution (pH > 5), the procedure having previously been validated by examination of the homologous xenon trioxide.
Avrorin et al. reported in 1982 that 212Fr compounds cocrystallised with their caesium analogues appeared to retain chemically bound radon after electron capture; analogies with xenon suggested the formation of RnO3, but this could not be confirmed.
[25] The standard electrode potential of the Rn2+/Rn couple has been estimated as +2.0 V,[38] although there is no evidence for the formation of stable radon ions or compounds in aqueous solution.
[44] 210Pb takes much longer to come in equilibrium with radon, dependent on environmental factors,[45] but if the environment permits accumulation of dust over extended periods of time, 210Pb and its decay products may contribute to overall radiation levels as well.
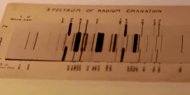
The likeness of the spectra of these three gases with those of argon, krypton, and xenon, and their observed chemical inertia led Sir William Ramsay to suggest in 1904 that the "emanations" might contain a new element of the noble-gas family.
[61] They wrote that "L'expression l'émanation du radium est fort incommode" ("the expression 'radium emanation' is very awkward") and suggested the new name niton (Nt) (from Latin: nitens, shining) to emphasize the radioluminescence property,[62] and in 1912 it was accepted by the International Commission for Atomic Weights.
[64] Even today, the word radon may refer to either the element or its isotope 222Rn, with thoron remaining in use as a short name for 220Rn to stem this ambiguity.
In 1530, Paracelsus described a wasting disease of miners, the mala metallorum, and Georg Agricola recommended ventilation in mines to avoid this mountain sickness (Bergsucht).
[67] In the US, studies and mitigation only followed decades of health effects on uranium miners of the Southwestern US employed during the early Cold War; standards were not implemented until 1971.
Beginning in the 1970s, research was initiated to address sources of indoor radon, determinants of concentration, health effects, and mitigation approaches.
While the average rate of production of 220Rn (from the thorium decay series) is about the same as that of 222Rn, the amount of 220Rn in the environment is much less than that of 222Rn because of the short half-life of 220Rn (55 seconds, versus 3.8 days respectively).
[82] The towns of Boulder, Montana; Misasa; Bad Kreuznach, Germany; and the country of Japan have radium-rich springs that emit radon.
[84] In 1971, Apollo 15 passed 110 km (68 mi) above the Aristarchus plateau on the Moon, and detected a significant rise in alpha particles thought to be caused by the decay of 222Rn.
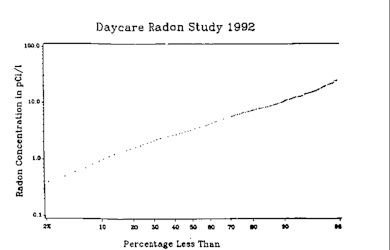
From 1975 up until 1984, small studies in Sweden, Austria, the United States and Norway aimed to measure radon indoors and in metropolitan areas.
[66] High concentrations of radon in homes were discovered by chance in 1984 after the stringent radiation testing conducted at the new Limerick Generating Station nuclear power plant in Montgomery County, Pennsylvania, United States revealed that Stanley Watras, a construction engineer at the plant, was contaminated by radioactive substances even though the reactor had never been fueled and Watras had been decontaminated each evening.
The second highest readings in Ireland were found in office buildings in the Irish town of Mallow, County Cork, prompting local fears regarding lung cancer.
[112] Inhalation therapy is carried out in Gasteiner-Heilstollen, Austria; Świeradów-Zdrój, Czerniawa-Zdrój, Kowary, Lądek-Zdrój, Poland; Harghita Băi, Romania; and Boulder, Montana.
[110][113] Radon has been produced commercially for use in radiation therapy, but for the most part has been replaced by radionuclides made in particle accelerators and nuclear reactors.
The gold layer keeps the radon within, and filters out the alpha and beta radiations, while allowing the gamma rays to escape (which kill the diseased tissue).
[122] As of 2009, it was under investigation as a possible earthquake precursor by NASA;[9] further research into the subject has suggested that abnormalities in atmospheric radon concentrations can be an indicator of seismic movement.
[126] Other X-ray sources such as 60Co and 192Ir became available after World War II and quickly replaced radium and thus radon for this purpose, being of lower cost and hazard.
During this period, several entrepreneurs opened former uranium mines in the US to the general public and advertised alleged health benefits from breathing radon gas underground.
Radon resulting from the high radium content in uncovered dumps and tailing ponds[3] can be easily released into the atmosphere and affect people living in the vicinity.
Empirical support from studies of the general population is inconsistent; a study of uranium miners found a correlation between radon exposure and chronic lymphocytic leukemia,[142] and current research supports a link between indoor radon exposure and poor health outcomes (i.e., an increased risk of lung cancer or childhood leukemia).
[149] The greatest risk of radon exposure arises in buildings that are airtight, insufficiently ventilated, and have foundation leaks that allow air from the soil into basements and dwelling rooms.
[154] One of the most comprehensive radon studies performed in the US by epidemiologist R. William Field and colleagues found a 50% increased lung cancer risk even at the protracted exposures at the EPA's action level of 4 pCi/L.
[157] Thoron is a minor contributor to the overall radiation dose received due to indoor radon exposure,[158] and can interfere with 222Rn measurements when not taken into account.
[13] Generally indoor radon can be mitigated by sub-slab depressurization and exhausting such radon-laden air to the outdoors, away from windows and other building openings.