Relativistic quantum chemistry
Relativistic quantum chemistry combines relativistic mechanics with quantum chemistry to calculate elemental properties and structure, especially for the heavier elements of the periodic table.
A prominent example is an explanation for the color of gold: due to relativistic effects, it is not silvery like most other metals.
[1] The term relativistic effects was developed in light of the history of quantum mechanics.
Initially, quantum mechanics was developed without considering the theory of relativity.
[2] Relativistic effects are those discrepancies between values calculated by models that consider relativity and those that do not.
[3] Relativistic effects are important for heavier elements with high atomic numbers, such as lanthanides and actinides.
[5] Beginning in 1935, Bertha Swirles described a relativistic treatment of a many-electron system,[6] despite Paul Dirac's 1929 assertion that the only imperfections remaining in quantum mechanics "give rise to difficulties only when high-speed particles are involved and are therefore of no importance in the consideration of the atomic and molecular structure and ordinary chemical reactions in which it is, indeed, usually sufficiently accurate if one neglects relativity variation of mass and velocity and assumes only Coulomb forces between the various electrons and atomic nuclei".
[7] Theoretical chemists by and large agreed with Dirac's sentiment until the 1970s, when relativistic effects were observed in heavy elements.
[9] Relativistic corrections were made to the Schrödinger equation (see Klein–Gordon equation) to describe the fine structure of atomic spectra, but this development and others did not immediately trickle into the chemical community.
Since atomic spectral lines were largely in the realm of physics and not in that of chemistry, most chemists were unfamiliar with relativistic quantum mechanics, and their attention was on lighter elements typical for the organic chemistry focus of the time.
[10] Dirac's opinion on the role relativistic quantum mechanics would play for chemical systems has been largely dismissed for two main reasons.
First, electrons in s and p atomic orbitals travel at a significant fraction of the speed of light.
Second, relativistic effects give rise to indirect consequences that are especially evident for d and f atomic orbitals.
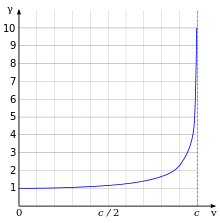
[8] One of the most important and familiar results of relativity is that the relativistic mass of the electron increases as
for a 1s electron, where v is its radial velocity, i.e., its instantaneous speed tangent to the radius of the atom.
If one substitutes the "relativistic mass" into the equation for the Bohr radius it can be written
At right, the above ratio of the relativistic and nonrelativistic Bohr radii has been plotted as a function of the electron velocity.
Notice how the relativistic model shows the radius decreases with increasing velocity.
This fits with intuition: electrons with lower principal quantum numbers will have a higher probability density of being nearer to the nucleus.
A nucleus with a large charge will cause an electron to have a high velocity.
Hg2(g) rarely forms and has a low dissociation energy, as expected due to the lack of strong bonds.
The relativistic contraction of the 6s2 orbital leads to gaseous mercury sometimes being referred to as a pseudo noble gas.
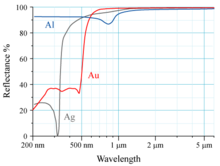
[11] The reflectivity of aluminium (Al), silver (Ag), and gold (Au) is shown in the graph to the right.
The human eye sees electromagnetic radiation with a wavelength near 600 nm as yellow.
An analogous transition occurs in silver, but the relativistic effects are smaller than in gold.
The golden color of caesium comes from the decreasing frequency of light required to excite electrons of the alkali metals as the group is descended.
For lithium through rubidium, this frequency is in the ultraviolet, but for caesium it reaches the blue-violet end of the visible spectrum; in other words, the plasmonic frequency of the alkali metals becomes lower from lithium to caesium.
Thus caesium transmits and partially absorbs violet light preferentially, while other colors (having lower frequency) are reflected; hence it appears yellowish.
[18] In Tl(I) (thallium), Pb(II) (lead), and Bi(III) (bismuth) complexes a 6s2 electron pair exists.
[8] Additional phenomena commonly caused by relativistic effects are the following: