Caesium auride
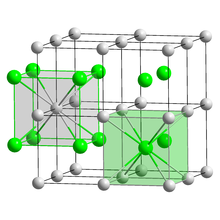
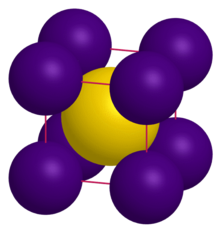
Caesium auride is the inorganic compound with the formula CsAu.
[2] CsAu is obtained by heating a stoichiometric mixture of caesium and gold.
The two metallic-yellow liquids react to give a transparent yellow product.
[3] Despite being a compound of two metals, CsAu lacks metallic properties since it is a salt with localized charges; it instead behaves as a semiconductor with band gap 2.6 eV.
[4] The compound hydrolyzes readily, yielding caesium hydroxide, metallic gold, and hydrogen.