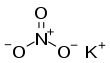Potassium nitrate
[5] Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks.
[6] In processed meats, potassium nitrate reacts with hemoglobin and myoglobin generating a red color, becoming highly toxic and carcinogenic.
As for nitrate, Egyptian and Hebrew words for it had the consonants n-t-r, indicating likely cognation in the Greek nitron, which was Latinised to nitrum or nitrium.
[20] Calcium nitrate, or lime saltpetre, was discovered on the walls of stables, from the urine of barnyard animals.
[21] The process involved burial of excrements (human or animal) in a field beside the nitraries, watering them and waiting until leaching allowed saltpeter to migrate to the surface by efflorescence.
[13]: 89, 311 [24] In 1561, Elizabeth I, Queen of England and Ireland, who was at war with Philip II of Spain, became unable to import saltpeter (of which the Kingdom of England had no home production), and had to pay "300 pounds gold" to the German captain Gerrard Honrik for the manual "Instructions for making saltpeter to growe" (the secret of the "Feuerwerkbuch" -the nitraries-).
The nitre beds were large rectangles of rotted manure and straw, moistened weekly with urine, "dung water", and liquid from privies, cesspools and drains, and turned over regularly.
The National Archives published payroll records that account for more than 29,000 people compelled to such labor in the state of Virginia.
The South was so desperate for saltpeter for gunpowder that one Alabama official reportedly placed a newspaper ad asking that the contents of chamber pots be saved for collection.
[28] He was writing with the express purpose of increasing production in the Confederate States to support their needs during the American Civil War.
On industrial scale it is prepared by the double displacement reaction between sodium nitrate and potassium chloride.
Potassium nitrate has an orthorhombic crystal structure at room temperature,[31] which transforms to a trigonal system at 128 °C (262 °F).
After that time, small arms and large artillery increasingly began to depend on cordite, a smokeless powder.
[36] It is also added to cigarettes to maintain an even burn of the tobacco[37] and is used to ensure complete combustion of paper cartridges for cap and ball revolvers.
[6] The use of potassium nitrate has been mostly discontinued because it gives slow and inconsistent results compared with sodium nitrite preparations such as "Prague powder" or pink "curing salt".
[46] In April 2023 the French Court of Appeals of Limoges confirmed that food-watch NGO Yuka was legally legitimate in describing Potassium Nitrate E249 to E252 as a "cancer risk", and thus rejected an appeal by the French charcuterie industry against the organisation.
[48][49] Potassium nitrate was once thought to induce impotence, and is still rumored to be in institutional food (such as military fare).
[citation needed] In One Flew Over the Cuckoo's Nest, Randle is asked by the nurses to take his medications, but not knowing what they are, he mentions he does not want anyone to "slip me saltpeter".
[67] In the Star Trek episode "Arena", Captain Kirk injures a gorn using a rudimentary cannon that he constructs using potassium nitrate as a key ingredient of gunpowder.
[citation needed] In 21 Jump Street, Jenko, played by Channing Tatum, gives a rhyming presentation about potassium nitrate for his chemistry class.
In Sharpe's Havoc, the French Captain Argenton laments that France needs to scrape its supply from cesspits.
The exact nature of "English salt" is a matter of debate, but it may have been a euphemism for potassium nitrate (saltpeter) due to its role in manufacturing gunpowder.