Samarium(II) chloride
Samarium(II) chloride (SmCl2) is a chemical compound, used as a radical generating agent in the ketone-mediated intraannulation reaction.
Reduction of samarium(III) chloride with samarium metal in a vacuum at a temperature of 800 °C to 900 °C, or with hydrogen gas at 350 °C yields samarium(II) chloride:[1] Samarium(II) chloride can also be prepared by reducing samarium(III) chloride with lithium metal/naphthalene in THF:[3] A similar reaction has been observed with sodium.
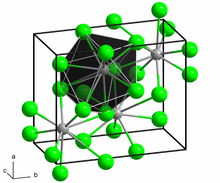
[2] Samarium(II) chloride adopts the PbCl2 (cotunnite) structure.
This inorganic compound–related article is a stub.
You can help Wikipedia by expanding it.