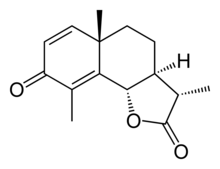
Santonin
It is a terpenoid and an organic compound consisting of colorless flat prisms, turning slightly yellow from the action of light and soluble in alcohol, chloroform and boiling water.
), santonin melts, and forms, if rapidly cooled, an amorphous mass, which instantly crystallizes oil coming in contact with a minute quantity of one of its solvents.
Santonin yields, with an alcoholic solution of potassium hydroxide, a bright pinkish-red liquid, which gradually becomes colorless.
Experiments in the 1880s showed that even after 40 hours, santonin had no lethal effect on roundworms using a saturated solution in dilute alkali.
[11] Santonin was formerly listed in U.S. and British pharmacopoeia, but it has fallen out of use with the development of safer ascaricides and is no longer registered as a drug in most countries.
[17] On the other hand, exposure to light in the solution phase results in the formation of monomeric skeletal rearrangement products.
[18] Santonin was developed in the 1830s by German chemists by extracting the chemical from Artemisia cina, a plant from Turkmenistan.
Santonin was used in treatment of infestation by the roundworm Ascaris lumbricoides and in ascarid parasitoses in general (including threadworm parasitosis).
It was sold in numerous formulations with varying degrees of effectiveness, such as worm lozenges, powders, syrups, and tonics.
[19] It was reported by an official of the Eastern & Russian Trading Company that during 1926, Japanese manufacturers were mixing santonin into nearly all pastry, confections, and tonics as part of a government-sponsored effort to eradicate intestinal parasites; Japan at the time imported five tons of santonin from Russia annually.
Commercial preparations containing santonin (usually containing a purgative laxative as well) also appeared in US drug formularies as late as the 1950s; the Modern Drug Encyclopedia and Therapeutic Index of 1955 listed Lumbricide (produced by Massengill) and a generic santonin preparation made by Winthrop-Stearns (now Winthrop-Sanofi).
Santonin was an agent that (compared to more modern anthelminthic drugs) was very complicated to use and entailed rather serious risk to the patient.
Large doses, however, produce toxic effects, aphasia, muscular tremors and epileptiform convulsions, and the disturbances of vision may go on to total blindness.
Among the toxic effects may be mentioned gastric pain, pallor and coldness of the surface, followed by heat and injection of the head, tremors, dizziness, pupillary dilatation, twitching of the eyes, stertor, copious sweating, hematuria, convulsive movements, tetanic cramps stupor, and insensibility.
Occasionally symptoms resembling cholera morbus have been produced, and in all cases the urine presents a characteristic yellowish or greenish-yellow hue.
Santonin often produces a singular effect upon the vision, causing surrounding objects to appear discolored, as if they were yellow or green, and occasionally blue or red; it also imparts a yellow or green color to the urine, and a reddish-purple color if that fluid be alkaline.
The view now held, however, is that of Rose, that the alkaline serum dissolves the santonin, which then acts upon the perspective centers of the brain, producing the chromatopsia or xanthopsia.
Due to the severe side effects (even when used as directed), the need for a purgative, and the development of many safer deworming drugs, santonin has largely fallen out of use.
[22] While absinthe is certainly more infamous for its content of thujone,[23] the liquor does also contain small amounts of santonin [citation needed] .