Secretion
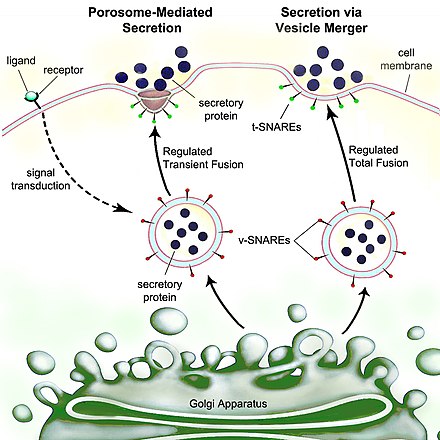
The classical mechanism of cell secretion is via secretory portals at the plasma membrane called porosomes.
Secretion is a very important mechanism in bacterial functioning and operation in their natural surrounding environment for adaptation and survival.
In the Golgi apparatus, the glycosylation of the proteins is modified and further post-translational modifications, including cleavage and functionalization, may occur.
The process begins as a leader sequence on the protein to be secreted is recognized by HlyA and binds HlyB on the membrane.
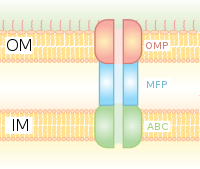
Type I secretion system transports various molecules, from ions, drugs, to proteins of various sizes (20 – 900 kDa).
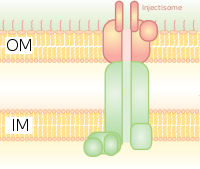
It is like a molecular syringe through which a bacterium (e.g. certain types of Salmonella, Shigella, Yersinia, Vibrio) can inject proteins into eukaryotic cells.
It was discovered in Agrobacterium tumefaciens, which uses this system to introduce the T-DNA portion of the Ti plasmid into the plant host, which in turn causes the affected area to develop into a crown gall (tumor).
Legionella pneumophila, the causing agent of legionellosis (Legionnaires' disease) utilizes a type IVB secretion system, known as the icm/dot (intracellular multiplication / defect in organelle trafficking genes) system, to translocate numerous effector proteins into its eukaryotic host.
T4SS also secrete virulence factor proteins directly into host cells as well as taking up DNA from the medium during natural transformation.
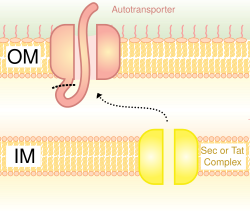
Proteins which use this pathway have the capability to form a beta-barrel with their C-terminus which inserts into the outer membrane, allowing the rest of the peptide (the passenger domain) to reach the outside of the cell.
[17] Type VI secretion systems were originally identified in 2006 by the group of John Mekalanos at the Harvard Medical School (Boston, USA) in two bacterial pathogens, Vibrio cholerae and Pseudomonas aeruginosa.
[18][19] These were identified when mutations in the Hcp and VrgG genes in Vibrio cholerae led to decreased virulence and pathogenicity.
Since then, Type VI secretion systems have been found in a quarter of all proteobacterial genomes, including animal, plant, human pathogens, as well as soil, environmental or marine bacteria.
[24] In addition to the use of the multiprotein complexes listed above, Gram-negative bacteria possess another method for release of material: the formation of bacterial outer membrane vesicles.
Vesicles from a number of bacterial species have been found to contain virulence factors, some have immunomodulatory effects, and some can directly adhere to and intoxicate host cells.
release of vesicles has been demonstrated as a general response to stress conditions, the process of loading cargo proteins seems to be selective.
[26] In some Staphylococcus and Streptococcus species, the accessory secretory system handles the export of highly repetitive adhesion glycoproteins.