Semaglutide
[14] In the US semaglutide (Wegovy) is indicated, in combination with a reduced calorie diet and increased physical activity, to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established cardiovascular disease and who are either obese or overweight;[16] to reduce excess body weight and maintain weight reduction long term in people aged twelve years of age and older with obesity or adults with overweight in the presence of at least one weight-related comorbid condition.
[37] The US Food and Drug Administration prescription label for semaglutide contains a boxed warning for thyroid C-cell tumors in rodents.
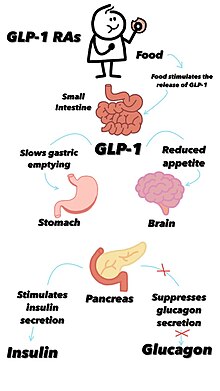
[27][41] Additionally, it inhibits the production of glucagon, the hormone that increases glycogenolysis (release of stored carbohydrate from the liver) and gluconeogenesis (synthesis of new glucose).
[27][46] In the 1970s, Jens Juul Holst and Joel Habener began research on the GLP-1 hormone, initially in relation to duodenal ulcer disease.
Their work, which later contributed significantly to diabetes and obesity treatments, earned them and Daniel J. Drucker the 2021 Warren Alpert Foundation Prize.
[47] Research continued and in 1993 Michael Nauck managed to infuse GLP-1 into people with type 2 diabetes, stimulating insulin while inhibiting glucagon and bringing blood glucose to normal levels.
However, treating diabetes patients with GLP-1 hormones resulted in significant side effects, leading researchers financed by Novo Nordisk to start looking to develop a suitable compound for therapeutic use.
[47] In 1998 a team of researchers at Novo Nordisk led by the scientist Lotte Bjerre Knudsen developed liraglutide, a glucagon-like peptide-1 receptor agonist that could be used to treat diabetes.
[48] In June 2008, a phase II clinical trial began studying semaglutide, a once-weekly diabetes therapy as a longer-acting alternative to liraglutide.
[23][51] The US Food and Drug Administration (FDA) approved semaglutide based on evidence from seven clinical trials of 4087 participants with type 2 diabetes.
[29] The FDA also considered data from one separate trial (NCT01720446[59]) of 3297 participants with type 2 diabetes who were at high risk for cardiovascular events.
[29] In March 2021, in a phase III randomized, double-blind trial, 1,961 adults with a body mass index of 30 or greater were assigned in a 2:1 ratio to a treatment with once-weekly subcutaneous semaglutide or placebo, plus lifestyle intervention.
[34] Participants in both groups also received standard-of-care medical treatment (e.g., management of blood pressure and cholesterol) and healthy lifestyle counseling (including diet and physical activity).
[65] In December 2017, the injectable version with the brand name Ozempic was approved for use by people with diabetes in the United States,[30][66] and, in January 2018, in Canada.
[18] In June 2021, a higher-dose version for injectable use sold under the brand name Wegovy was approved by the FDA as an anti-obesity medication for long-term weight management in adults.
[19][22] In January 2023, the US FDA prescription label for Rybelsus was updated to reflect that it can be used as a first-line treatment for adults with type 2 diabetes.
)[83][84] In the US, Wegovy has a list price of $1,349.02 per month as of 2022, suggesting that because of the high costs many people "who could most benefit from weight loss may be unable to afford such expensive drugs".
[89] By 2023, Novo Nordisk had become the most valuable corporation in the European Union, worth more than US$500 billion, and accounted for almost all recent economic growth in Denmark.
[105] French national health care insurance system database had previously suggested that one to three years of use of glucagon-like peptide-1 receptor agonists like exenatide, liraglutide, and dulaglutide may be linked with increased occurrence of thyroid cancer.
A meta-analysis involving data from 37 randomized controlled trials and 19 real-world studies (46,719 patients) showed that semaglutide use over 18 months was not associated with increased risks of any cancer, supported by a high grade of evidence.
[111] In January 2024, a preliminary review conducted by the US Food and Drug Administration (FDA) confirmed no evidence had been found to suggest that the medicine causes suicidal thoughts or actions.
[116][117] Novo Nordisk reported that its oral medication, Rybelsus, reduced the risk of cardiovascular events by 14% in a late-stage trial.
[122] Researchers analyzed three years of electronic medical records from over 1 million patients with Type 2 diabetes who had not been previously diagnosed with Alzheimer's and had at least one additional cardiometabolic risk factor.
[123] The study found that, compared to seven other anti-diabetic drugs, semaglutide was particularly effective in lowering the risk of Alzheimer's, as well as other GLP-1 medications in people.
[125] The 2024 research analyzed the records of nearly 228,000 people in Sweden from 2006 until 2023 and noticed that those using semaglutide or liraglutide were significantly less likely to face alcohol-related hospitalizations.