Meristem
They contribute to the formation of structures such as fruits, leaves, and seeds, as well as supportive tissues like stems and roots.
The growth and proliferation rates of cells vary within the meristem, with higher activity at the periphery compared to the central region.
The term meristem was first used in 1858 by Swiss botanist Carl Wilhelm von Nägeli (1817–1891) in his book Beiträge zur Wissenschaftlichen Botanik ("Contributions to Scientific Botany").
SAM and RAM cells divide rapidly and are considered indeterminate, in that they do not possess any defined end status.
[citation needed] Shoot apical meristems are the source of all above-ground organs, such as leaves and flowers.
[citation needed] Primordia of leaves, sepals, petals, stamens, and ovaries are initiated here at the rate of one every time interval, called a plastochron.
[14] CLV1 acts to promote cellular differentiation by repressing WUS activity outside of the central zone containing the stem cells.
Type-B ARRs work as transcription factors to activate genes downstream of cytokinin, including A-ARRs.
[16] Therefore, A-ARRs do not contribute to the activation of transcription, and by competing for phosphates from phosphotransfer proteins, inhibit B-ARRs function.
[19] As a result, B-ARRs are no longer inhibited, causing sustained cytokinin signaling in the center of the shoot apical meristem.
Evidence suggests that the QC maintains the surrounding stem cells by preventing their differentiation, via signal(s) that are yet to be discovered.
Recent findings indicate that QC can also act as a reservoir of stem cells to replenish whatever is lost or damaged.
In angiosperms, intercalary (sometimes called basal) meristems occur in monocot (in particular, grass) stems at the base of nodes and leaf blades.
Intercalary meristems are capable of cell division, and they allow for rapid growth and regrowth of many monocots.
[citation needed] Recent investigations into apical dominance and the control of branching have revealed a new plant hormone family termed strigolactones.
[27] These studies suggest that the regulation of stem cell number, identity and differentiation might be an evolutionarily conserved mechanism in monocots, if not in angiosperms.
Rice also contains another genetic system distinct from FON1-FON2, that is involved in regulating stem cell number.
Members of the KNOX family have been found in plants as diverse as Arabidopsis thaliana, rice, barley and tomato.
For example, among members of Antirrhineae, only the species of the genus Antirrhinum lack a structure called spur in the floral region.
Researchers carried out transposon mutagenesis in Antirrhinum majus, and saw that some insertions led to formation of spurs that were very similar to the other members of Antirrhineae,[28] indicating that the loss of spur in wild Antirrhinum majus populations could probably be an evolutionary innovation.
The growth of nitrogen-fixing root nodules on legume plants such as soybean and pea is either determinate or indeterminate.
Thus, soybean (or bean and Lotus japonicus) produce determinate nodules (spherical), with a branched vascular system surrounding the central infected zone.
In contrast, nodules on pea, clovers, and Medicago truncatula are indeterminate, to maintain (at least for some time) an active meristem that yields new cells for Rhizobium infection.
[33] Meristems may also be induced in the roots of legumes such as soybean, Lotus japonicus, pea, and Medicago truncatula after infection with soil bacteria commonly called Rhizobia.
[citation needed] Cells of the inner or outer cortex in the so-called "window of nodulation" just behind the developing root tip are induced to divide.
The critical signal substance is the lipo-oligosaccharide Nod factor, decorated with side groups to allow specificity of interaction.
The Nod factor receptor proteins NFR1 and NFR5 were cloned from several legumes including Lotus japonicus, Medicago truncatula and soybean (Glycine max).
This process involves a leaf-vascular tissue located LRR receptor kinases (LjHAR1, GmNARK and MtSUNN), CLE peptide signalling, and KAPP interaction, similar to that seen in the CLV1,2,3 system.
LjKLAVIER also exhibits a nodule regulation phenotype though it is not yet known how this relates to the other AON receptor kinases.
These cork cells are impermeable to water and gases because of a substance called suberin that coats them.
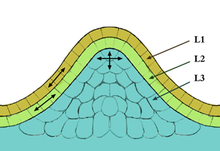

- Central zone
- Peripheral zone
- Medullary (i.e. central) meristem
- Medullary tissue

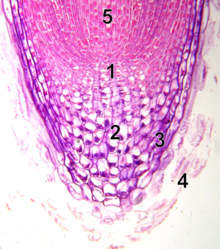
- quiescent center
- calyptrogen (live rootcap cells)
- rootcap
- sloughed off dead rootcap cells
- procambium

