Silicate
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula [SiO(4-2x)−4−x]n, where 0 ≤ x < 2.
For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass).
In single-chain silicates, which are a type of inosilicate, tetrahedra link to form a chain by sharing two oxygen atoms each.
Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each.
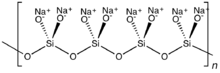
In this group, known as phyllosilicates, tetrahedra all share three oxygen atoms each and in turn link to form two-dimensional sheets.
Silicates with alkali cations and small or chain-like anions, such as sodium ortho- and metasilicate, are fairly soluble in water.
Silicates of non-alkali cations, or with sheet and tridimensional polymeric anions, generally have negligible solubility in water at normal conditions.
In a typical preparation, monomeric orthosilicate was found to react completely in 75 seconds; dimeric pyrosilicate in 10 minutes; and higher oligomers in considerably longer time.
[8] The nature of soluble silicates is relevant to understanding biomineralization and the synthesis of aluminosilicates, such as the industrially important catalysts called zeolites.
So, geopolymer cements could contribute to limiting the CO2 emissions in the Earth atmosphere and the global warming caused by this greenhouse gas.

4