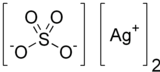Silver sulfate
Silver sulfate is the inorganic compound with the formula Ag2SO4.
It is a white solid with low solubility in water.
Silver sulfate precipitates as a solid when an aqueous solution of silver nitrate is treated with sulfuric acid: It is purified by recrystallization from concentrated sulfuric acid, a step that expels traces of nitrate.
[8] The synthesis of silver(II) sulfate (AgSO4) with a divalent silver ion instead of a monovalent silver ion was first reported in 2010[9] by adding sulfuric acid to silver(II) fluoride (HF escapes).
It is a black solid that decomposes exothermically at 120 °C with evolution of oxygen and the formation of the pyrosulfate.