Silver tetrafluoroborate
It is a white solid, although commercial samples often are gray, that dissolves in polar organic solvents as well as water.
A simple route entails dissolving silver carbonate in aqueous tetrafluoroboric acid.
[3] It can also be produced by treating silver(I) fluoride with boron trifluoride in nitromethane solution.
[4] In the inorganic and organometallic chemistry laboratory, silver tetrafluoroborate, sometimes referred to "silver BF-4", is a used as a reagent to remove halide ligands and to oxidize electron-rich complexes.
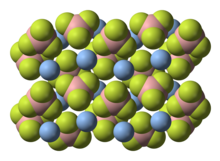
According to X-ray crystallography, the solid compound consists of Ag+ centers bound to four fluoride sites of the BF4−.