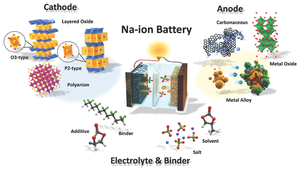
Sodium-ion battery
In some cases, its working principle and cell construction are similar to those of lithium-ion battery (LIB) types, but it replaces lithium with sodium as the intercalating ion.
SIBs received academic and commercial interest in the 2010s and early 2020s, largely due to lithium's high cost, uneven geographic distribution, and environmentally-damaging extraction process.
A downside of the larger ionic radius of Na+ is a slower intercalation kinetics of sodium-ion electrode materials.
Several companies such as HiNa and CATL in China, Faradion in the United Kingdom, Tiamat in France, Northvolt in Sweden,[5] and Natron Energy in the US, are close to achieving the commercialization of NIBs, with the aim of employing sodium layered transition metal oxides (NaxTMO2), Prussian white (a Prussian blue analogue[6]) or vanadium phosphate as cathode materials.
However, CATL, the world's biggest lithium-ion battery manufacturer, announced in 2022 the start of mass production of SIBs.
[citation needed] Due to the physical and electrochemical properties of sodium, SIBs require different materials from those used for LIBs.
[20] Hard carbon was the preferred choice of Faradion due to its excellent combination of capacity, (lower) working potentials, and cycling stability.
[25] After sodium adsorption, a carbon arsenide anode maintains structural stability at 300 K, indicating long cycle life.
[26] Wang, et al. reported that a self-regulating alloy interface of nickel antimony (NiSb) was chemically deposited on Na metal during discharge.
Unfortunately, the formation of such alloys is usually accompanied by a large volume change, which in turn results in the pulverization (crumbling) of the material after a few cycles.
For example, with tin sodium forms an alloy Na15Sn4, which is equivalent to 847 mAh/g specific capacity, with a resulting enormous volume change up to 420%.
This combination significantly increased volumetric energy density and eliminated capacity fade in half cells.
[36] Some other materials, such as mercury, electroactive polymers and sodium terephthalate derivatives,[37] have also been demonstrated in laboratories, but did not provoke commercial interest.
These oxides typically have a higher tap density and a lower electronic resistivity, than other posode materials (such as phosphates).
[4] A P2-type Na2/3Fe1/2Mn1/2O2 oxide from earth-abundant Fe and Mn resources can reversibly store 190 mAh/g at average discharge voltage of 2.75 V vs Na/Na+ utilising the Fe3+/4+ redox couple – on par or better than commercial lithium-ion cathodes such as LiFePO4 or LiMn2O4.
On the other hand, a stronger covalent bonding of the polyanion positively impacts cycle life and safety and increases the cell voltage.
[47] A French startup TIAMAT develops Na+ ion batteries based on a sodium-vanadium-phosphate-fluoride cathode material Na3V2(PO4)2F3, which undergoes two reversible 0.5 e-/V transitions: at 3.2V and at 4.0 V.[48] A startup from Singapore, SgNaPlus is developing and commercialising Na3V2(PO4)2F3 cathode material, which shows very good cycling stability, utilising the non-flammable glyme-based electrolyte.
Such high specific charges are rarely observed only in PBA samples with a low number of structural defects.
[75][better source needed] Altris AB was founded by Associate Professor Reza Younesi, his former PhD student, Ronnie Mogensen, and Associate Professor William Brant as a spin-off from Uppsala University, Sweden,[76] launched in 2017 as part of research efforts from the team on sodium-ion batteries.
The research was conducted at the Ångström Advanced Battery Centre led by Prof. Kristina Edström at Uppsala University.
The company offers a proprietary iron-based Prussian blue analogue for the positive electrode in non-aqueous sodium-ion batteries that use hard carbon as the anode.
[77] Altris holds patents on non-flammable fluorine-free electrolytes consisting of NaBOB in alkyl-phosphate solvents, Prussian white cathode, and cell production.
In 2023, they invested $1.4B USD into the construction of a sodium-ion battery plant in Xuzhou with an annual output of 30 GWh.
This battery pack features an expected range of over 400 kilometres (250 mi), 4C fast charging capability, the ability to be discharged at −40 °C (−40 °F), and no difference to the driving experience at −20 °C (−4 °F).
[81] On November 18th 2024, CATL announced its second generation sodium-ion battery to be released in 2025 and reach mass market by 2027.
In 2023, HiNa partnered with JAC as the first company to put a sodium-ion battery in an electric car, the Sehol E10X.
[94] In 2019, it was reported that HiNa installed a 100 kWh sodium-ion battery energy storage system in East China.
It has been developed in cooperation with Pune's Indian Institute of Science Education and Research over the course of almost a decade and claims several notable benefits over existing alternatives such as lead-acid and lithium-ion.
Among its standout features are a longer lifespan of 3,000–6,000 cycles, faster charging than traditional batteries, greater resistance to below-freezing temperatures and with varied energy densities between 100 and 170 Wh/Kg.
[98][99][100] Natron Energy, a spin-off from Stanford University, uses Prussian blue analogues for both cathode and anode with an aqueous electrolyte.