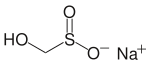
Rongalite
Addition of at least one equivalent of formaldehyde pushes the equilibrium towards the side of the adduct and reacts further to give the bis-(hydroxymethyl)sulfone.
Sodium hydroxymethanesulfinate was originally developed in the early 20th century for the textile industry as a shelf-stable source of sulfoxylate ion, where the latter can be generated at will.
Occasionally, alkylation will occur also at oxygen, thus xylylene dibromide gives both the sulfone and the isomeric sulfinate ester.
A niche use is its use as water conditioner for aquaria as it rapidly reduces chlorine and chloramine and reacts with ammonia to form the innocuous aminomethylsulfinate ion.
The compound has been used increasingly in commercial cosmetic hair dye colour removers despite the generation of formaldehyde, a known human carcinogen.