Ammonia
Pliny, in Book XXXI of his Natural History, refers to a salt named hammoniacum, so called because of the proximity of its source to the Temple of Jupiter Amun (Greek Ἄμμων Ammon) in the Roman province of Cyrenaica.
Its most conspicuous property is its ability to dissolve alkali metals to form highly coloured, electrically conductive solutions containing solvated electrons.
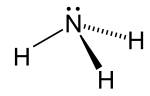
The ammonia molecule readily undergoes nitrogen inversion at room temperature; a useful analogy is an umbrella turning itself inside out in a strong wind.
Nitrogen oxides can be formed as kinetic products in the presence of appropriate catalysts, a reaction of great industrial importance in the production of nitric acid: A subsequent reaction leads to NO2: The combustion of ammonia in air is very difficult in the absence of a catalyst (such as platinum gauze or warm chromium(III) oxide), due to the relatively low heat of combustion, a lower laminar burning velocity, high auto-ignition temperature, high heat of vapourization, and a narrow flammability range.
[42][clarification needed] For studying the kinetics of ammonia combustion, knowledge of a detailed reliable reaction mechanism is required, but this has been challenging to obtain.
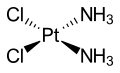
Ammine complexes of chromium(III) formed the basis of Alfred Werner's revolutionary theory on the structure of coordination compounds.
Werner noted only two isomers (fac- and mer-) of the complex [CrCl3(NH3)3] could be formed, and concluded the ligands must be arranged around the metal ion at the vertices of an octahedron.
[61] At a later period, when sal ammoniac was obtained by distilling the hooves and horns of oxen and neutralizing the resulting carbonate with hydrochloric acid, the name 'spirit of hartshorn' was applied to ammonia.
[21][62] Gaseous ammonia was first isolated by Joseph Black in 1756 by reacting sal ammoniac (ammonium chloride) with calcined magnesia (magnesium oxide).
Anhydrous ammonia is widely used in industrial refrigeration applications and hockey rinks because of its high energy efficiency and low cost.
The potential importance of ammonia as a refrigerant has increased with the discovery that vented CFCs and HFCs are potent and stable greenhouse gases.
The process was deemed effective and safe by the US Department of Agriculture based on a study that found that the treatment reduces E. coli to undetectable levels.
If the process of creating it can be scaled up via purely renewable resources, producing green ammonia, it could make a major difference in avoiding climate change.
[112][113] In June 2022, IHI Corporation succeeded in reducing greenhouse gases by over 99% during combustion of liquid ammonia in a 2,000-kilowatt-class gas turbine achieving truly CO2-free power generation.
[114] In July 2022, Quad nations of Japan, the U.S., Australia and India agreed to promote technological development for clean-burning hydrogen and ammonia as fuels at the security grouping's first energy meeting.
[116] Nitrous oxide may also be a problem as it is a "greenhouse gas that is known to possess up to 300 times the Global Warming Potential (GWP) of carbon dioxide".
[129] Ammonia is regulated in the US as a non-flammable gas, but it meets the definition of a material that is toxic by inhalation and requires a hazardous safety permit when transported in quantities greater than 3,500 US gallons (13,000 L; 2,900 imp gal).
[137] At lower concentrations, around 0.05 mg/L, un-ionised ammonia is harmful to fish species and can result in poor growth and feed conversion rates, reduced fecundity and fertility and increase stress and susceptibility to bacterial infections and diseases.
[141] Experts warn that ammonium compounds not be allowed to come in contact with bases (unless in an intended and contained reaction), as dangerous quantities of ammonia gas could be released.
Experts also warn that prolonged contact of ammonia solutions with silver, mercury or iodide salts can also lead to explosive products: such mixtures are often formed in qualitative inorganic analysis, and that it needs to be lightly acidified but not concentrated (<6% w/v) before disposal once the test is completed.
[152] Beyond simply mediating proton transfer to the nitrogen reduction reaction, ethanol has been found to play a multifaceted role, influencing electrolyte transformations and contributing to the formation of the solid electrolyte interphase, which enhances overall reaction efficiency[153][154] In 1994, Tsuneto et al. used lithium electrodeposition in tetrahydrofuran to synthesize ammonia at more moderate pressures with reasonable Faradaic efficiency.
Hyperammonemia contributes to the confusion and coma of hepatic encephalopathy, as well as the neurological disease common in people with urea cycle defects and organic acidurias.
In birds, reptiles, and terrestrial snails, metabolic ammonium is converted into uric acid, which is solid and can therefore be excreted with minimal water loss.
[171] Ammonia has been detected in the atmospheres of the giant planets Jupiter, Saturn, Uranus and Neptune, along with other gases such as methane, hydrogen, and helium.
[180] All other proposed formation reactions have rate constants of between two and 13 orders of magnitude smaller, making their contribution to the abundance of ammonia relatively insignificant.
A comparison of emission line widths indicates that turbulent or systematic velocities do not increase in the central cores of molecular clouds.
The hot gas has temperatures above 70 K, which was inferred from ammonia line ratios and appears to be closely associated with the innermost portions of the nuclear bar seen in CO.[190] NH3 was also monitored by VLA toward a sample of four galactic ultracompact HII regions: G9.62+0.19, G10.47+0.03, G29.96-0.02, and G31.41+0.31.
Based upon temperature and density diagnostics, it is concluded that in general such clumps are probably the sites of massive star formation in an early evolutionary phase prior to the development of an ultracompact HII region.
This premise can be applied to dark clouds, regions suspected of having extremely low temperatures and possible sites for future star formation.
Doppler-shifted velocity components allow for the separation of distinct regions of molecular gas that can trace outflows and hot cores originating from forming stars.