Sonogashira coupling
It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide.
The Sonogashira cross-coupling reaction has been employed in a wide variety of areas, due to its usefulness in the formation of carbon–carbon bonds.
[1] Specific examples include its use in the synthesis of tazarotene,[2] which is a treatment for psoriasis and acne, and in the preparation of SIB-1508Y, also known as Altinicline,[3] a nicotinic receptor agonist.
The alkynylation reaction of aryl halides using aromatic acetylenes was reported in 1975 in three independent contributions by Cassar,[4] Dieck and Heck[5] as well as Sonogashira, Tohda and Hagihara.
A rapid development of the Pd/Cu systems followed and enabled myriad synthetic applications, while Cassar-Heck conditions were left, maybe unjustly, all but forgotten.
[12] Until recently, the exact mechanism by which the Cu-free reaction occurs was under debate, with critical mechanistic questions unanswered.
Depending on the rate of the competition between amine and phosphines, a dynamic and complex interplay is expected when using different coordinative bases.
In addition, deaerated conditions are formally needed for Sonogashira coupling reactions because the palladium(0) complexes are unstable in the air, and oxygen promotes the formation of homocoupled acetylenes.
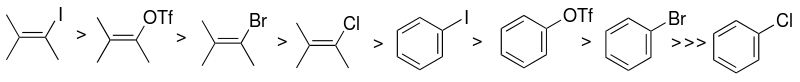
[7] The choice of aryl halide or pseudohalide substrate (sp2-carbon) is one of the factors that mainly influence the reactivity of the Sonogashira catalytic system.
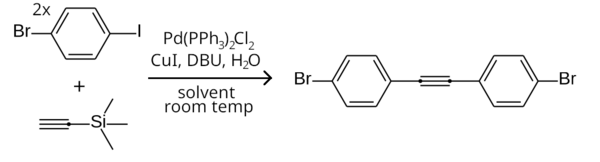
[9] An example is the symmetrical Sonogashira coupling of two equivalents of 1-bromo-4-iodobenzene with trimethylsilylacetylene (with the trimethylsilyl group removed in-situ) to form bis(4-bromophenyl)acetylene.
It has been discovered that secondary amines such as piperidine, morpholine, or diisopropylamine in particular can react efficiently and reversibly with trans–RPdX(PPh3)2 complexes by substituting one PPh3 ligand.
It may also be removed using DBU in situ, allowing the monosubstituted acetylene to react further with another aryl halide to form diphenylacetylene and derivatives.
This leads to what is known as the Glaser coupling reaction, which is an undesired formation of homocoupling products of acetylene derivatives upon oxidation.
[26] In some cases stoichiometric amounts of silver oxide can be used in place of CuI for copper-free Sonogashira couplings.
While the copper-free mechanism has been shown to be viable, attempts to incorporate the various transition metals mentioned above as less expensive alternatives to palladium catalysts have shown a poor track record of success due to contamination of the reagents with trace amounts of palladium, suggesting that these theorized pathways are extremely unlikely, if not impossible, to achieve.
[34] A highly efficient gold and palladium combined methodology for the Sonogashira coupling of a wide array of electronically and structurally diverse aryl and heteroaryl halides has been reported.
[35] The orthogonal reactivity of the two metals shows high selectivity and extreme functional group tolerance in Sonogashira coupling.
The issues dealing with recovery of the often expensive catalyst after product formation poses a serious drawback for large-scale applications of homogeneous catalysis.
[9] Structures known as metallodendrimers combine the advantages of homogeneous and heterogeneous catalysts, as they are soluble and well defined on the molecular level, and yet they can be recovered by precipitation, ultrafiltration, or ultracentrifugation.
[36] Some recent examples can be found about the use of dendritic palladium complex catalysts for the copper-free Sonogashira reaction.
[37] The dendrimeric catalysts could usually be recovered by simple precipitation and filtration and reused up to five times, with diminished activity produced by dendrimer decomposition and not by palladium leaching being observed.
Pyridines and pyrimidines have shown good complexation properties for palladium and have been employed in the formation of catalysts suitable for Sonogashira couplings.
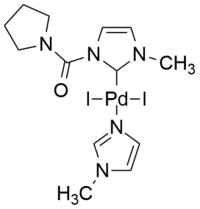
The dipyrimidyl-palladium complex shown below has been employed in the copper-free coupling of iodo-, bromo-, and chlorobenzene with phenylacetylene using N-butylamine as base in THF solvent at 65 °C.
The success of normal NHCs is greatly attributed to their superior σ-donating capabilities as compared to phosphines, which is even greater in abnormal NHC counterparts.
Employed as ligands in palladium complexes, NHCs contributed greatly to the stabilization and activation of precatalysts and have therefore found application in many areas of organometallic homogeneous catalysis, including Sonogashira couplings.
An efficient, cationic palladium catalyst of PEPPSI type, i.e., iPEPPSI (internal pyridine-enhanced precatalyst preparation stabilization and initiation) was demonstrated to efficiently catalyse the copper-free Sonogashira reaction in water as the only solvent, under aerobic conditions, in the absence of copper, amines, phosphines and other additives.
The list of cases where the typical Sonogashira reaction using aryl halides has been employed is large, and choosing illustrative examples is difficult.
[48] Several of the most recent and promising applications of this coupling methodology toward the total synthesis of natural products exclusively employed the typical copper-cocatalyzed reaction.
The synthesis of these natural products involved the use of Sonogashira cross-coupling to build the carbon backbone of each molecule.
[citation needed] It can be derived from vinylic systems and terminal acetylenes by using a configuration-retention stereospecific procedure such as the Sonogashira reaction.










![Mechanism for the Cu-free Sonogashira reaction.[13][14]](http://upload.wikimedia.org/wikipedia/commons/thumb/9/90/Cu-free-mechanism.png/642px-Cu-free-mechanism.png)