Alkane
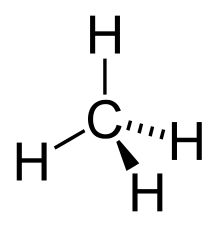
In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single.
Methane is produced by methanogenic bacteria and some long-chain alkanes function as pheromones in certain animal species or as protective waxes in plants and fungi.
Unbranched, saturated hydrocarbon chains are named systematically with a Greek numerical prefix denoting the number of carbons and the suffix "-ane".
[5] In 1866, August Wilhelm von Hofmann suggested systematizing nomenclature by using the whole sequence of vowels a, e, i, o and u to create suffixes -ane, -ene, -ine (or -yne), -one, -une, for the hydrocarbons CnH2n+2, CnH2n, CnH2n−2, CnH2n−4, CnH2n−6.
[6] In modern nomenclature, the first three specifically name hydrocarbons with single, double and triple bonds;[7] while "-one" now represents a ketone.
Although this is not strictly necessary and is not part of the IUPAC naming system, the usage is still common in cases where one wishes to emphasize or distinguish between the straight-chain and branched-chain isomers, e.g., "n-butane" rather than simply "butane" to differentiate it from isobutane.
[18] Two factors influence the strength of the van der Waals forces: Under standard conditions, from CH4 to C4H10 alkanes are gaseous; from C5H12 to C17H36 they are liquids; and after C18H38 they are solids.
[18] On the other hand, cycloalkanes tend to have higher boiling points than their linear counterparts due to the locked conformations of the molecules, which give a plane of intermolecular contact.
However, alkanes' melting points follow a more complex pattern, due to variations in the properties of their solid crystals.
The carbon-13 resonances depend on the number of hydrogen atoms attached to the carbon: δC = 8–30 (primary, methyl, –CH3), 15–55 (secondary, methylene, –CH2–), 20–60 (tertiary, methyne, C–H) and quaternary.
Since alkanes have high ionization energies, their electron impact mass spectra show weak currents for their molecular ions.
The general equation for complete combustion is: In the absence of sufficient oxygen, carbon monoxide or even soot can be formed, as shown below: For example, methane: See the alkane heat of formation table for detailed data.
There are three steps: Experiments have shown that all halogenation produces a mixture of all possible isomers, indicating that all hydrogen atoms are susceptible to reaction.
An example can be seen in the monobromination of propane:[18] In the Reed reaction, sulfur dioxide and chlorine convert hydrocarbons to sulfonyl chlorides under the influence of light.
In both types of processes, the corresponding reactive intermediates (radicals, ions) are permanently regenerated, and thus they proceed by a self-propagating chain mechanism.
Chemical analysis showed that the abundances of ethane and methane were roughly equal, which is thought to imply that its ices formed in interstellar space, away from the Sun, which would have evaporated these volatile molecules.
Traces of methane gas (about 0.0002% or 1745 ppb) occur in the Earth's atmosphere, produced primarily by methanogenic microorganisms, such as Archaea in the gut of ruminants.
[18] Natural gas contains primarily methane and ethane, with some propane and butane: oil is a mixture of liquid alkanes and other hydrocarbons.
Energy is released by the oxidation of hydrogen: It is probable that our current deposits of natural gas were formed in a similar way.
The fungus Amorphotheca resinae prefers the longer-chain alkanes in aviation fuel, and can cause serious problems for aircraft in tropical regions.
The exact composition of the layer of wax is not only species-dependent but also changes with the season and such environmental factors as lighting conditions, temperature or humidity.
[40] The Jeffrey pine is noted for producing exceptionally high levels of n-heptane in its resin, for which reason its distillate was designated as the zero point for one octane rating.
[41] Emission of gaseous and volatile alkanes such as ethane, pentane, and hexane by plants has also been documented at low levels, though they are not generally considered to be a major component of biogenic air pollution.
[42] Edible vegetable oils also typically contain small fractions of biogenic alkanes with a wide spectrum of carbon numbers, mainly 8 to 35, usually peaking in the low to upper 20s, with concentrations up to dozens of milligrams per kilogram (parts per million by weight) and sometimes over a hundred for the total alkane fraction.
[44] One example, in which both plant and animal alkanes play a role, is the ecological relationship between the sand bee (Andrena nigroaenea) and the early spider orchid (Ophrys sphegodes); the latter is dependent for pollination on the former.
Sand bees use pheromones in order to identify a mate; in the case of A. nigroaenea, the females emit a mixture of tricosane (C23H48), pentacosane (C25H52) and heptacosane (C27H56) in the ratio 3:3:1, and males are attracted by specifically this odor.
In industry, the main substrates are organonitrogen and organosulfur impurities, i.e. the heteroatoms are N and S. The specific processes are called hydrodenitrification and hydrodesulfurization: Hydrogenolysis can be applied to the conversion of virtually any functional group into hydrocarbons.
Partial combustion of coal and related solid organic compounds generates carbon monoxide, which can be hydrogenated using the Fischer–Tropsch process.
However, the higher melting points of these alkanes can cause problems at low temperatures and in polar regions, where the fuel becomes too thick to flow correctly.
In the latter function, they work at the same time as anti-corrosive agents, as their hydrophobic nature means that water cannot reach the metal surface.