Spectroelectrochemistry
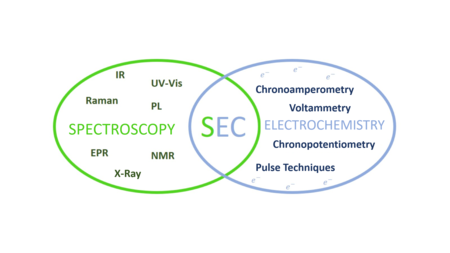
Spectroelectrochemistry (SEC) is a set of multi-response analytical techniques in which complementary chemical information (electrochemical and spectroscopic) is obtained in a single experiment.
[6] The main objective of spectroelectrochemical experiments is to obtain simultaneous, time-resolved and in-situ electrochemical and spectroscopic information on reactions taking place on the electrode surface.
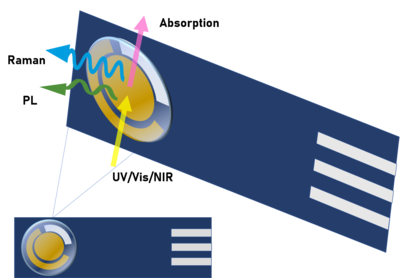
[11][12] Photoluminescence (PL) is a phenomenon related to the ability of some compounds that, after absorbing specific electromagnetic radiation, relax to a lower energy state through emission of photons.
X-rays can originate absorption, emission or scattering phenomena, allowing to perform both quantitative and qualitative analysis depending on the phenomenon taking place.
Its combination with electrochemical techniques can provide detailed and quantitative information about the functional groups, topology, dynamics and the three-dimensional structure of molecules in solution during a charge transfer process.
[15] The physical principles of this technique are analogous to those of NMR, but in the case of EPR, electronic spins are excited instead of nuclear, that is interesting in certain electrode reactions.
The versatility of spectroelectrochemistry is increasing due to the possibility of using several electrochemical techniques in different spectral regions depending on the purpose of the study and the information of interest.