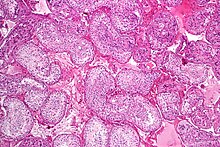
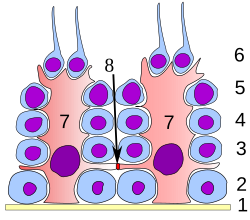
Spermatogenesis
This process starts with the mitotic division of the stem cells located close to the basement membrane of the tubules.
[4] It starts during puberty and usually continues uninterrupted until death, although a slight decrease can be discerned in the quantity of produced sperm with increase in age (see Male infertility).
[6] Clinically, small fluctuations in temperature such as from an athletic support strap, causes no impairment in sperm viability or count.
In the following table, ploidy, copy number and chromosome/chromatid counts are for one cell, generally prior to DNA synthesis and division (in G1 if applicable).
Spermatocytogenesis is the male form of gametocytogenesis and results in the formation of spermatocytes possessing half the normal complement of genetic material.
Instead, spermatogonial stem cells divide mitotically to produce copies of themselves, ensuring a constant supply of spermatogonia to fuel spermatogenesis.
[16] The anterior part of the tail (called midpiece) thickens because mitochondria are arranged around the axoneme to ensure energy supply.
The DNA is packaged firstly with specific nuclear basic proteins, which are subsequently replaced with protamines during spermatid elongation.
The mature spermatozoa are released from the protective Sertoli cells into the lumen of the seminiferous tubule in a process called spermiation.
The non-motile spermatozoa are transported to the epididymis in testicular fluid secreted by the Sertoli cells with the aid of peristaltic contraction.
However, transport of the mature spermatozoa through the remainder of the male reproductive system is achieved via muscle contraction rather than the spermatozoon's recently acquired motility.
A single Sertoli cell extends from the basement membrane to the lumen of the seminiferous tubule, although the cytoplasmic processes are difficult to distinguish at the light microscopic level.
Sertoli cells serve a number of functions during spermatogenesis, they support the developing gametes in the following ways: The intercellular adhesion molecules ICAM-1 and soluble ICAM-1 have antagonistic effects on the tight junctions forming the blood-testis barrier.
[clarification needed][23] Dietary deficiencies (such as vitamins B, E and A), anabolic steroids, metals (cadmium and lead), x-ray exposure, dioxin, alcohol, and infectious diseases will also adversely affect the rate of spermatogenesis.
In humans the mechanism is not completely understood; however it is known that initiation of spermatogenesis occurs at puberty due to the interaction of the hypothalamus, pituitary gland and Leydig cells.
[27] In contrast to FSH, luteinizing hormone (LH) appears to have little role in spermatogenesis outside of inducing gonadal testosterone production.
[27][28] FSH stimulates both the production of androgen binding protein (ABP) by Sertoli cells, and the formation of the blood-testis barrier.
Intratesticular testosterone levels are 20–100 or 50–200 times higher than the concentration found in blood, although there is variation over a 5- to 10-fold range amongst healthy men.
Studies from rodent models suggest that gonadotropins (both LH and FSH) support the process of spermatogenesis by suppressing the proapoptotic signals and therefore promote spermatogenic cell survival.
[34] Levels of estrogen that are too high can be detrimental to spermatogenesis due to suppression of gonadotropin secretion and by extension intratesticular testosterone production.
[28][36] Disorders of spermatogenesis may cause oligospermia, which is semen with a low concentration of sperm[37] and is a common finding in male infertility.