Square planar molecular geometry
In chemistry, the square planar molecular geometry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds.
The noble gas compound xenon tetrafluoride adopts this structure as predicted by VSEPR theory.
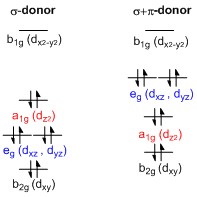
A general d-orbital splitting diagram for square planar (D4h) transition metal complexes can be derived from the general octahedral (Oh) splitting diagram, in which the dz2 and the dx2−y2 orbitals are degenerate and higher in energy than the degenerate set of dxy, dxz and dyz orbitals.
It bears electron density on the x- and y-axes and therefore interacts with the filled ligand orbitals.
The dxy, dxz and dyz orbitals are generally presented as degenerate but they have to split into two different energy levels with respect to the irreducible representations of the point group D4h.