Standard addition
This method is useful for analyzing complex samples where a matrix effect interferes with the analyte signal.
Standard addition involves adding known amounts of analyte to an unknown sample, a process known as spiking.
[1] This approach was the first reported use of standard addition, and was introduced by a German mining chemist, Hans Hohn, in 1937.
[3] In his polarography practical book, titled Chemische Analysen mit dem Polargraphen, Hohn referred this method as Eizhusatzes, which translates to "calibration addition" in English.
Later in the German literature, this method was called as Standardzugabe, meaning "standard addition" in English.
To apply this method, analysts prepare multiple solutions containing equal amounts of unknown and spike them with varying concentrations of the analyte.
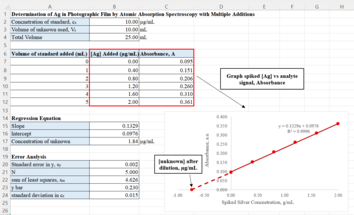
[4] Suppose an analyst is determining the concentration of silver in samples of waste solution in photographic film by atomic absorption spectroscopy.
Matrix effects occur even with methods such as plasma spectrometry, which have a reputation for being relatively free from interferences.
This x-intercept represents the silver concentration of the test sample where there is no standard solution added.
[7] These effects are caused by other substances present in the unknown sample that are often independent of the analyte concentration.
[5] Analysts also needs to evaluate the precision of the determined unknown concentration by calculating for the standard deviation,