Stem-cell niche
[citation needed] A Nature Insight review defines niche as follows: "Stem-cell populations are established in 'niches' — specific anatomic locations that regulate how they participate in tissue generation, maintenance and repair.
It constitutes a basic unit of tissue physiology, integrating signals that mediate the balanced response of stem cells to the needs of organisms.
The interplay between stem cells and their niche creates the dynamic system necessary for sustaining tissues, and for the ultimate design of stem-cell therapeutics ...
A similar dependence of self-renewal potential on proximity to the niche border was reported in the context of hair follicle, in an in vivo live-imaging study.
[5] This bi-compartmental structure of stem cell niche has been mathematically modeled to obtain the optimal architecture that leads to the maximum delay in double-hit mutant production.
[7] Mathematical modeling of the intestinal gland reveals that the small population size within the stem cell niche minimizes the probability of carcinogenesis occurring anywhere, at the expense of gradually accumulated deleterious mutations throughout organismal lifetime—a process that contributes to tissue degradation and aging.
[8] Therefore, the population size of the stem cell niche represents an evolutionary trade-off between the probability of cancer formation and the rate of aging.
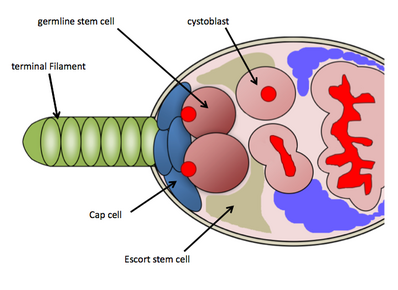
In particular, the GSC niche is well studied in the genetic model organism Drosophila melanogaster and has provided an extensive understanding of the molecular basis of stem cell regulation.
[11] Because of the abundant genetic tools available for use in Drosophila melanogaster and the ease of detecting GSCs through histological stainings, researchers have uncovered several molecular pathways controlling GSC maintenance and activity.
[14] BMP signalling in the niche functions to directly repress expression of Bag-of-marbles (Bam) in GSCs, which is up-regulated in developing cystoblast cells.
[16] In the germarium, BMP signaling has a short-range effect, therefore the physical attachment of GSCs to cap cells is important for maintenance and activity.
The Drosophila testis GSC niche has proven a valuable model system for examining a wide range of cellular processes and signalling pathways.
[27] The process of spermatogenesis begins when the GSCs divide asymmetrically, producing a GSC that maintains hub contact, and a gonialblast that exits the niche.
[28][29] This leads to activation of the Drosophila STAT, Stat92E, a transcription factor which effects GSC adhesion to the hub cells,[30] and SSC self-renewal via Zfh-1.
These ligands are secreted into the GSCs from the SSCs and hub cells, activate BMP signalling, and suppress the expression of Bam, a differentiation factor.
[41] In addition the blood testis barrier provides architectural support and is composed of tight junction components such as occludins, claudins and zonula occludens (ZOs) which show dynamic expression during spermatogenesis.
[42] For example, claudin 11 has been shown to be a necessary component of these tight junctions as mice lacking this gene have a defective blood testis barrier and do not produce mature spermatozoa.
[40] GDNF (Glial cell-derived neurotrophic factor) is known to stimulate self-renewal of SSCs and is secreted by the sertoli cells under the influence of gonadotropin FSH.
GDNF is a related member of the TGFβ superfamily of growth factors and when overexpressed in mice, an increase in undifferentiated spermatogonia was observed which led to the formation of germ tumours.
[35][40] In corroboration for its role as a renewal factor, heterozygous knockout male mice for GDNF show decreased spermatogenesis that eventually leads to infertility.
FGF2 (Fibroblast growth factor −2), secreted by sertoli cells, has also been shown to influence the renewal of SSCs and undifferentiated spermatogonia in a similar manner to GDNF.
[35] Plzf (Promyelocytic leukaemia zinc finger) has also been implicated in regulating SSC self-renewal and is expressed by Asingle, Apaired and Aaligned spermatogonia.
Some important signals in the hair follicle stem cell niche produced by the mesenchymal dermal papilla or the bulge include BMP, TGF-β and Fibroblast growth factor (FGF) ligands and Wnt inhibitors.
They are composed of Isl1+/Flk1+ cardiac progenitor cells (CPCs) that are localized into discrete clusters within a ColIV and laminin extracellular matrix (ECM).
Immunohistochemical staining has been used to demonstrate that differentiating CPCs, which migrate away from the progenitor clusters and into the ColI and fibronectin ECM surrounding the niche, down-regulate Isl1 while up-regulating mature cardiac markers such as troponin C.[51] There is a current controversy over the role of Isl1+ cells in the cardiovascular system.
[70] Epithelial–mesenchymal transition is a morphogenetic process, normally occurs in embryogenesis that is "hijacked" by cancer stem cells by detaching from their primary place and migrating to another one.
This process is regulated by CSCs microenvironment via the same signalling pathways as in embryogenesis using the growth factors (TGF-β, PDGF, EGF), cytokine IL-8 and extracellular matrix components.
[citation needed] During injury, support cells are able to activate a program for repair, recapitulating aspects of development in the area of damage.
For instance in the CNS, injury is able to activate a developmental program in astrocytes that allow them to express molecules that support stem cells such as chemokines i.e. SDF-1[75] and morphogens such as sonic hedgehog.
[76] It is evident that biophysio-chemical characteristics of ECM such as composition, shape, topography, stiffness, and mechanical strength can control the stem cell behavior.