Cancer stem cell
Existing cancer treatments have mostly been developed based on animal models, where therapies able to promote tumor shrinkage were deemed effective.
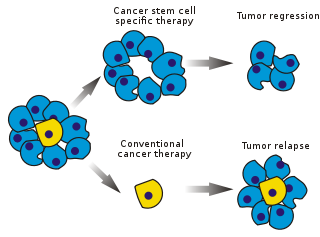
As CSCs form a small proportion of the tumor, this may not necessarily select for drugs that act specifically on the stem cells.
[2] The term itself was coined in a highly cited paper in 2001 by biologists Tannishtha Reya, Sean J. Morrison, Michael F. Clarke and Irving Weissman.
However, certain perspectives maintain that this demarcation is artificial, since both processes act in complementary manners as far as actual tumor populations are concerned.
However, both stem cells[17] and CSCs[18] possess unique immunological properties which render them highly resistant towards immunosurveillance.
Confounding this debate is the discovery that many cancer cells demonstrate a phenotypic plasticity under therapeutic challenge, altering their transcriptomes to a more stem-like state to escape destruction.
Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro.
Supporters of the CSC paradigm argue that only a small fraction of the injected cells, the CSCs, have the potential to generate a tumor.
CSCs have recently been identified in several solid tumors, including: Once the pathways to cancer are hypothesized, it is possible to develop predictive mathematical models,[42] e.g., based on the cell compartment method.
Such a model predicted that repeated insult to mature cells increases the formation of abnormal progeny and the risk of cancer.
In other words, fully differentiated cell undergoes mutations or extracellular signals that drive it back to a stem-like state.
A tumor hosts several types of stem cells, one optimal to the specific environment and other less successful lines.
[51] CSCs can also be identified by efflux of incorporated Hoechst dyes via multidrug resistance (MDR) and ATP-binding cassette (ABC) Transporters.
As with normal stem cells, the CSCs isolated from brain or prostate tumors also have the ability to form anchor-independent spheres.
[52] Recent years have seen an advent of genetic approaches to identify cancer stem cells in experimental rodents.
In such studies, following the induction of cancer (usually through the application of mutagens), a genetic cassette is activated resulting in the expression of an easily identifiable marker, for instance green fluorescent protein (GFP).
While former theory dictates the role of genetic, epigenetic and micro environment where tumour cell resides to acquire undifferentiated tumorigenic traits.
Commonly, markers specific for normal stem cells are used for isolating CSCs from solid and hematological tumors.
[86] EMT's important feature is the loss of membrane E-cadherin in adherens junctions, where β-catenin may play a significant role.
[95] The two-phase expression pattern hypothesis proposes two forms of cancer stem cells - stationary (SCS) and mobile (MCS).
[96]CSCs have implications for cancer therapy, including for disease identification, selective drug targets, prevention of metastasis and intervention strategies.
[99] In 2009, scientists identified the compound salinomycin, which selectively reduces the proportion of breast CSCs in mice by more than 100-fold relative to Paclitaxel, a commonly used chemotherapeutic agent.
[56] It has also been found that CSCs have the ability to exacerbate drug resistance through overexpression of ABC transporter proteins that can pump hydrophobic compounds.
[110] The design of new drugs for targeting CSCs requires understanding the cellular mechanisms that regulate cell proliferation.
The first advances in this area were made with hematopoietic stem cells (HSCs) and their transformed counterparts in leukemia, the disease for which the origin of CSCs is best understood.
A normal stem cell may be transformed into a CSC through dysregulation of the proliferation and differentiation pathways controlling it or by inducing oncoprotein activity.
The Polycomb group transcriptional repressor Bmi-1 was discovered as a common oncogene activated in lymphoma[111] and later shown to regulate HSCs.
A branch of the Notch signaling pathway that involves the transcription factor Hes3 regulates a number of cultured cells with CSC characteristics obtained from glioblastoma patients.
DMAPT, a water-soluble derivative of parthenolide, induces oxidative stress and inhibits NF-κB signaling[122] for AML (leukemia) and possibly myeloma and prostate cancer.
LF3 strongly inhibits this binding in vitro, in cell lines and reduced tumor growth in mouse models.