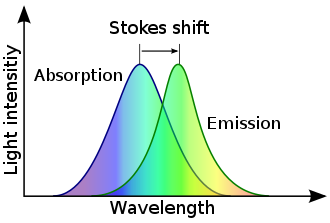
Stokes shift
Stokes shift is the difference (in energy, wavenumber or frequency units) between positions of the band maxima of the absorption and emission spectra (fluorescence and Raman being two examples) of the same electronic transition.
When a fluorophore enters an excited state, its dipole moment changes, but surrounding solvent molecules cannot adjust so quickly.
Analyzing the intensity and frequency of the spectral shift provides valuable information about the vibrational modes of molecules, enabling the identification of chemical bonds, functional groups, and molecular conformations.
This unique property makes it particularly valuable in various technological fields, including security printing, anti-counterfeiting measures, and luminescent displays.
By harnessing anti-Stokes fluorescence, this pigment enables the creation of vibrant and durable inks, coatings, and materials with enhanced visibility and authentication capabilities.
[11] In direct-bandgap thin-film semiconducting layers Stokes shifted emission can originate from three main sources: doping, strain, and disorder.