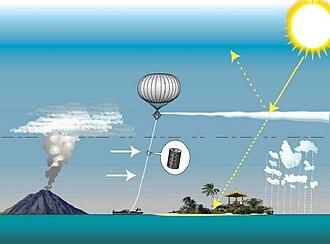
Stratospheric aerosol injection
[14][15][13] Inorganic aerosols are mainly produced when sulfur dioxide reacts with water vapor to form gaseous sulfuric acid and various salts (often through an oxidation reaction in the clouds), which are then thought to experience hygroscopic growth and coagulation and then shrink through evaporation[16][14] as microscopic liquid droplets or fine (diameter of about 0.1 to 1.0 micrometre) sulfate solid particles in a colloidal suspension,[17][15] with smaller particles at times coagulating into larger ones.
[17] And sometimes, aerosols are produced from photochemical decomposition of COS (carbonyl sulfide), or when solid sulfates in the sea salt spray can react with gypsum dust particles).
[19] Volcanic emissions vary significantly in composition, and have complex chemistry due to the presence of ash particulates and a wide variety of other elements in the plume.
[20] However, before the Industrial Revolution, dimethyl sulfide pathway was the largest contributor to sulfate aerosol concentrations in a more average year with no major volcanic activity.
[25] The discovery of these negative effects spurred the rush to reduce atmospheric sulfate pollution, typically through flue-gas desulfurization installations at power plants, such as wet scrubbers or fluidized bed combustion.
[41] Similarly, India's sulfur dioxide emissions appear to have been largely flat in the 2010s, as more coal-fired power plants were fitted with pollution controls even as the newer ones were still coming online.
[42] Yet, around the time these treaties and technology improvements were taking place, evidence was coming in that sulfate aerosols were affecting both the visible light received by the Earth and its surface temperature.
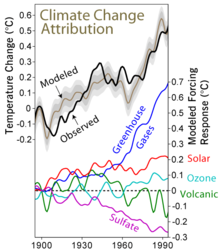
[47] On the other hand, multiple studies have shown that between 1950s and 1980s, the amount of sunlight reaching the surface declined by around 4–5% per decade,[48][49][50] even though the changes in solar radiation at the top of the atmosphere were never more than 0.1-0.3%.
[51] Yet, this trend (commonly described as global dimming) began to reverse in the 1990s, consistent with the reductions in anthropogenic sulfate pollution,[52][53][54] while at the same time, climate change accelerated.
Formation of the aerosols and their effects on the atmosphere can be studied in the lab, with methods like ion-chromatography and mass spectrometry[60] Samples of actual particles can be recovered from the stratosphere using balloons or aircraft,[61] and remote satellites were also used for observation.
[1] Deploying in the stratosphere ensures that the aerosols are at their most effective, and that the progress of clean air measures would not be reversed: more recent research estimated that even under the highest-emission scenario RCP 8.5, the addition of stratospheric sulfur required to avoid 4 °C (7.2 °F) relative to now (and 5 °C (9.0 °F) relative to the preindustrial) would be effectively offset by the future controls on tropospheric sulfate pollution, and the amount required would be even less for less drastic warming scenarios.
"[70] One study calculated the impact of injecting sulfate particles, or aerosols, every one to four years into the stratosphere in amounts equal to those lofted by the volcanic eruption of Mount Pinatubo in 1991,[71] but did not address the many technical and political challenges involved in potential solar geoengineering efforts.
[79][80] Concentration of precursor injection in a single longitude appears to be beneficial, with condensation onto existing particles reduced, giving better control of the size distribution of aerosols resulting.
[81] The long residence time of carbon dioxide in the atmosphere may require a millennium-timescale commitment to aerosol injection[82] if aggressive emissions abatement is not pursued simultaneously.
However, at about $18 billion per year per degree Celsius of warming avoided (in 2020 USD), a solar geoengineering program with substantial climate impact would lie well beyond the financial reach of individuals, small states, or other non-state potential rogue actors.
Notably, both solar radiation management and climate change (as well as greenhouse gases) could satisfy the definition of "air pollution" which the signatories commit to reduce, depending on their actual negative effects.
Full implementation or large scale climate response field tests of stratospheric sulfate aerosols could cause countries to exceed their limits.
However, because stratospheric injections would be spread across the globe instead of concentrated in a few nearby countries, and could lead to net reductions in the "air pollution" which the CLRTAP Convention is to reduce so they may be allowed.
[144][145] Sir David King, a former chief scientific adviser to the government of the United Kingdom, stated that SCoPEX and Gates' plans to dim the sun with calcium carbonate could have disastrous effects.
[146] In 2012, the Bristol University-led Stratospheric Particle Injection for Climate Engineering (SPICE) project planned on a limited field test to evaluate a potential delivery system.
The group received support from the EPSRC, NERC and STFC to the tune of £2.1 million[147] and was one of the first UK projects aimed at providing evidence-based knowledge about solar radiation management.
[151] Mikhail Budyko is believed to have been the first, in 1974, to put forth the concept of artificial solar radiation management with stratospheric sulfate aerosols if global warming ever became a pressing issue.